当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Pressure CO Electroreduction at Silver Produces Ethanol and Propanol
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-30 , DOI: 10.1002/anie.202108902 Stefan J Raaijman 1 , Maarten P Schellekens 2 , Paul J Corbett 2 , Marc T M Koper 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-30 , DOI: 10.1002/anie.202108902 Stefan J Raaijman 1 , Maarten P Schellekens 2 , Paul J Corbett 2 , Marc T M Koper 1
Affiliation
|
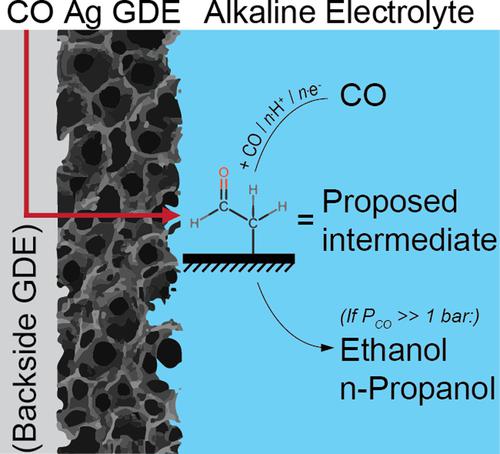
Reducing CO2 to long-chain carbon products is attractive considering such products are typically more valuable than shorter ones. However, the best electrocatalyst for making such products from CO2, copper, lacks selectivity. By studying alternate C2+ producing catalysts we can increase our mechanistic understanding, which is beneficial for improving catalyst performance. Therefore, we investigate CO reduction on silver, as density functional theory (DFT) results predict it to be good at forming ethanol. To address the current disagreement between DFT and experimental results (ethanol vs. no ethanol), we investigated CO reduction at higher surface coverage (by increasing pressure) to ascertain if desorption effects can explain the discrepancy. In terms of product trends, our results agree with the DFT-proposed acetaldehyde-like intermediate, yielding ethanol and propanol as C2+ products—making the CO2 electrochemistry of silver very similar to that of copper at sufficiently high coverage.
中文翻译:

银的高压 CO 电还原产生乙醇和丙醇
考虑到此类产品通常比较短的产品更有价值,将 CO 2还原为长链碳产品是有吸引力的。然而,用于从 CO 2铜制造此类产品的最佳电催化剂缺乏选择性。通过学习替代 C 2+生产催化剂,我们可以增加对机理的理解,这有利于提高催化剂的性能。因此,我们研究了银上的 CO 还原,因为密度泛函理论 (DFT) 结果预测它擅长形成乙醇。为了解决当前 DFT 和实验结果(乙醇与无乙醇)之间的分歧,我们研究了在较高表面覆盖率下(通过增加压力)的 CO 减少,以确定解吸效应是否可以解释这种差异。在产品趋势方面,我们的结果与 DFT 提出的类乙醛中间体一致,产生乙醇和丙醇作为 C 2+产品——在足够高的覆盖率下,银的 CO 2电化学与铜的 CO 2电化学非常相似。
更新日期:2021-09-20
中文翻译:

银的高压 CO 电还原产生乙醇和丙醇
考虑到此类产品通常比较短的产品更有价值,将 CO 2还原为长链碳产品是有吸引力的。然而,用于从 CO 2铜制造此类产品的最佳电催化剂缺乏选择性。通过学习替代 C 2+生产催化剂,我们可以增加对机理的理解,这有利于提高催化剂的性能。因此,我们研究了银上的 CO 还原,因为密度泛函理论 (DFT) 结果预测它擅长形成乙醇。为了解决当前 DFT 和实验结果(乙醇与无乙醇)之间的分歧,我们研究了在较高表面覆盖率下(通过增加压力)的 CO 减少,以确定解吸效应是否可以解释这种差异。在产品趋势方面,我们的结果与 DFT 提出的类乙醛中间体一致,产生乙醇和丙醇作为 C 2+产品——在足够高的覆盖率下,银的 CO 2电化学与铜的 CO 2电化学非常相似。































 京公网安备 11010802027423号
京公网安备 11010802027423号