当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New chemiluminescent method of levofloxacin and ofloxacin determination based on terbium (III)-sensitized fluoroquinolone–KBrO3 reaction
Luminescence ( IF 3.2 ) Pub Date : 2021-07-29 , DOI: 10.1002/bio.4128
Małgorzata Kaczmarek 1 , Krzysztof Staninski 1 , Mikołaj Stodolny 2
Luminescence ( IF 3.2 ) Pub Date : 2021-07-29 , DOI: 10.1002/bio.4128
Małgorzata Kaczmarek 1 , Krzysztof Staninski 1 , Mikołaj Stodolny 2
Affiliation
|
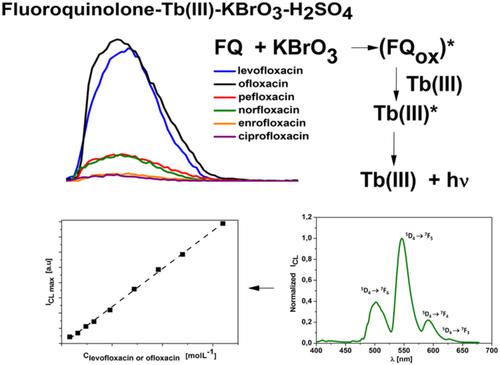
Fluoroquinolones can be oxidized with some agents, in this study selected fluoroquinolones (levofloxacin, ofloxacin, ciprofloxacin, norfloxacin, pefloxacin and enrofloxacin) were oxidized with potassium bromate in the presence of terbium (III) ions. According to the kinetic and spectral analysis of chemiluminescence emitted by the above systems, the terbium (III) ions were the only emitter. The excitation of the lanthanide ion was a result of the process of energy transfer from the products of fluoroquinolones oxidation to Tb(III) ions. The highest intensity of chemiluminescence was obtained for levofloxacin and ofloxacin containing an alkoxy substituent at C-8 in the quinoline ring. The chemiluminescence intensity was correlated linearly (r = 0.9994) with the concentration of ofloxacin (or levofloxacin) in the range 1 × 10−6 to 4 × 10−5 mol L−1; the detection limit was 3 × 10−7 mol L−1 for both fluoroquinolones. In the optimized conditions, the chemiluminescence of the levofloxacin (or ofloxacin)–Tb(III)–KBrO3–H2SO4 systems was used to determine these compounds in a mixture of fluoroquinolones and in pharmaceuticals.
中文翻译:

基于铽(III)敏化氟喹诺酮-KBrO3反应的左氧氟沙星和氧氟沙星化学发光新方法
氟喹诺酮类药物可以被某些试剂氧化,本研究中选择的氟喹诺酮类药物(左氧氟沙星、氧氟沙星、环丙沙星、诺氟沙星、培氟沙星和恩诺沙星)在铽 (III) 离子存在下用溴酸钾氧化。根据上述系统发出的化学发光的动力学和光谱分析,铽(III)离子是唯一的发射体。镧系元素离子的激发是从氟喹诺酮类氧化产物到 Tb(III) 离子的能量转移过程的结果。左氧氟沙星和氧氟沙星在喹啉环的 C-8 处含有烷氧基取代基,获得了最高强度的化学发光。化学发光强度呈线性相关(r = 0.9994) 氧氟沙星(或左氧氟沙星)的浓度范围为 1 × 10 -6至 4 × 10 -5 mol L -1;两种氟喹诺酮类药物的检测限均为 3 × 10 -7 mol L -1。在优化的条件下,左氧氟沙星(或氧氟沙星)–Tb(III)–KBrO 3 –H 2 SO 4系统的化学发光用于测定氟喹诺酮类混合物和药物中的这些化合物。
更新日期:2021-07-29
中文翻译:

基于铽(III)敏化氟喹诺酮-KBrO3反应的左氧氟沙星和氧氟沙星化学发光新方法
氟喹诺酮类药物可以被某些试剂氧化,本研究中选择的氟喹诺酮类药物(左氧氟沙星、氧氟沙星、环丙沙星、诺氟沙星、培氟沙星和恩诺沙星)在铽 (III) 离子存在下用溴酸钾氧化。根据上述系统发出的化学发光的动力学和光谱分析,铽(III)离子是唯一的发射体。镧系元素离子的激发是从氟喹诺酮类氧化产物到 Tb(III) 离子的能量转移过程的结果。左氧氟沙星和氧氟沙星在喹啉环的 C-8 处含有烷氧基取代基,获得了最高强度的化学发光。化学发光强度呈线性相关(r = 0.9994) 氧氟沙星(或左氧氟沙星)的浓度范围为 1 × 10 -6至 4 × 10 -5 mol L -1;两种氟喹诺酮类药物的检测限均为 3 × 10 -7 mol L -1。在优化的条件下,左氧氟沙星(或氧氟沙星)–Tb(III)–KBrO 3 –H 2 SO 4系统的化学发光用于测定氟喹诺酮类混合物和药物中的这些化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号