当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origin of Performances of Pt/Cu Single-Atom Alloy Catalysts for Propane Dehydrogenation
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-07-28 , DOI: 10.1021/acs.jpcc.1c04295 Shijia Sun 1, 2 , Guodong Sun 1, 2 , Chunlei Pei 1, 2 , Zhi-Jian Zhao 1, 2 , Jinlong Gong 1, 2, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-07-28 , DOI: 10.1021/acs.jpcc.1c04295 Shijia Sun 1, 2 , Guodong Sun 1, 2 , Chunlei Pei 1, 2 , Zhi-Jian Zhao 1, 2 , Jinlong Gong 1, 2, 3
Affiliation
|
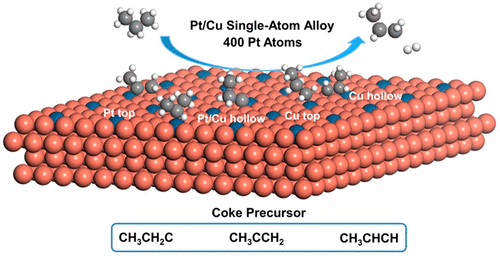
The propane dehydrogenation (PDH) reaction converts cheap propane to value-added propene. Pt-based catalysts show high performance in PDH, but suffer from coke formation and deactivation. Therefore, promoter, that is, a second metal component, is required to enhance its stability. Our previous study has constructed Pt/Cu single atom alloy (SAA) catalysts and achieved high PDH selectivity and anticoke ability. However, the nature of its high performance in PDH still remains to be revealed. This paper describes the origin of catalytic performance for Pt/Cu SAA in PDH via density functional theory (DFT) calculations and kinetic Monte Carlo (kMC) simulations. We constructed a complex reaction network with 54 reversible reaction steps, including adsorption, desorption, C–H bond breaking, and C–C bond cracking processes on the Pt/Cu SAA catalyst. The high selectivity of propene has been demonstrated because of the higher occurrence of propene formation and, simultaneously, the high energy barriers for deep dehydrogenation of propene. The lower coverages of the coke species origin from the deep dehydrogenation instead of the C–C bond cracking for Pt/Cu SAA catalyst, which is different from that proposed for Pt catalyst. The simulation suggests that hydrogen (H2) cofeeding can further reduce the surface coke species. Overall, the current study provides fundamental insights into the origin of high selectivity and anticoke ability to help the design of stable and high-performance Pt-based catalysts.
中文翻译:

用于丙烷脱氢的 Pt/Cu 单原子合金催化剂性能的起源
丙烷脱氢 (PDH) 反应将廉价的丙烷转化为具有附加值的丙烯。Pt 基催化剂在 PDH 中表现出高性能,但会形成焦炭和失活。因此,需要促进剂,即第二金属组分来增强其稳定性。我们之前的研究已经构建了 Pt/Cu 单原子合金 (SAA) 催化剂,并实现了高 PDH 选择性和抗焦能力。然而,其在 PDH 中的高性能的本质仍有待揭示。本文通过密度泛函理论 (DFT) 计算和动力学蒙特卡罗 (kMC) 模拟描述了 Pt/Cu SAA 在 PDH 中催化性能的起源。我们构建了一个复杂的反应网络,具有 54 个可逆反应步骤,包括在 Pt/Cu SAA 催化剂上的吸附、解吸、C-H 键断裂和 C-C 键裂解过程。丙烯的高选择性已被证明,因为丙烯形成的发生率较高,同时丙烯深度脱氢的高能垒。焦炭物种的较低覆盖率源于深度脱氢而不是 Pt/Cu SAA 催化剂的 C-C 键裂化,这与 Pt 催化剂提出的不同。模拟表明氢(H2)共进料可以进一步减少表面焦炭种类。总体而言,目前的研究为高选择性和抗焦能力的起源提供了基本见解,以帮助设计稳定和高性能的 Pt 基催化剂。
更新日期:2021-09-02
中文翻译:

用于丙烷脱氢的 Pt/Cu 单原子合金催化剂性能的起源
丙烷脱氢 (PDH) 反应将廉价的丙烷转化为具有附加值的丙烯。Pt 基催化剂在 PDH 中表现出高性能,但会形成焦炭和失活。因此,需要促进剂,即第二金属组分来增强其稳定性。我们之前的研究已经构建了 Pt/Cu 单原子合金 (SAA) 催化剂,并实现了高 PDH 选择性和抗焦能力。然而,其在 PDH 中的高性能的本质仍有待揭示。本文通过密度泛函理论 (DFT) 计算和动力学蒙特卡罗 (kMC) 模拟描述了 Pt/Cu SAA 在 PDH 中催化性能的起源。我们构建了一个复杂的反应网络,具有 54 个可逆反应步骤,包括在 Pt/Cu SAA 催化剂上的吸附、解吸、C-H 键断裂和 C-C 键裂解过程。丙烯的高选择性已被证明,因为丙烯形成的发生率较高,同时丙烯深度脱氢的高能垒。焦炭物种的较低覆盖率源于深度脱氢而不是 Pt/Cu SAA 催化剂的 C-C 键裂化,这与 Pt 催化剂提出的不同。模拟表明氢(H2)共进料可以进一步减少表面焦炭种类。总体而言,目前的研究为高选择性和抗焦能力的起源提供了基本见解,以帮助设计稳定和高性能的 Pt 基催化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号