当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Achieving C(sp2)–C(sp3) Coupling with BCP-F2 Building Blocks via Barluenga Coupling: A Comparative Approach
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.joc.1c01370 Xiaoshen Ma 1 , Charles S Yeung 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-07-27 , DOI: 10.1021/acs.joc.1c01370 Xiaoshen Ma 1 , Charles S Yeung 1
Affiliation
|
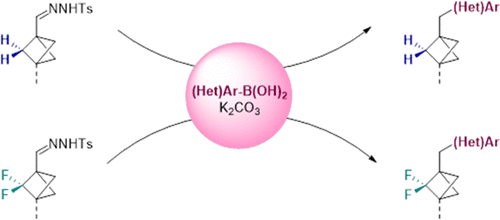
We report our efforts toward achieving C(sp2)–C(sp3) coupling reactions with 2,2-difluorobicyclo[1.1.1]pentane (BCP-F2) building blocks. By comparing the reactivities of matching pairs of bicyclo[1.1.1]pentane (BCP) and BCP-F2 analogues, we discovered that the Barluenga coupling reaction was the only cross-coupling protocol that translated well between the two structural motifs in contrast to other reported protocols. In this chemistry, a BCP-F2 bearing a tosylhydrazone functional group is cross-coupled with an arylboronic acid. These results further expanded the scope of BCP-F2 building blocks for potential applications in organic chemistry as well as medicinal chemistry.
中文翻译:

通过 Barluenga 耦合实现 C(sp2)–C(sp3) 与 BCP-F2 构建块的耦合:一种比较方法
我们报告了我们为实现 C(sp 2 )–C(sp 3 ) 偶联反应与 2,2-二氟双环[1.1.1] 戊烷 (BCP-F 2 ) 构建单元所做的努力。通过比较双环 [1.1.1] 戊烷 (BCP) 和 BCP-F 2类似物匹配对的反应性,我们发现 Barluenga 偶联反应是唯一一种在两种结构基序之间转换良好的交叉偶联方案,与其他报告的协议。在该化学中,带有甲苯磺酰腙官能团的 BCP-F 2与芳基硼酸交叉偶联。这些结果进一步扩展了 BCP-F 2构建块的范围,用于有机化学和药物化学的潜在应用。
更新日期:2021-08-07
中文翻译:

通过 Barluenga 耦合实现 C(sp2)–C(sp3) 与 BCP-F2 构建块的耦合:一种比较方法
我们报告了我们为实现 C(sp 2 )–C(sp 3 ) 偶联反应与 2,2-二氟双环[1.1.1] 戊烷 (BCP-F 2 ) 构建单元所做的努力。通过比较双环 [1.1.1] 戊烷 (BCP) 和 BCP-F 2类似物匹配对的反应性,我们发现 Barluenga 偶联反应是唯一一种在两种结构基序之间转换良好的交叉偶联方案,与其他报告的协议。在该化学中,带有甲苯磺酰腙官能团的 BCP-F 2与芳基硼酸交叉偶联。这些结果进一步扩展了 BCP-F 2构建块的范围,用于有机化学和药物化学的潜在应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号