当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystallinity Effect of NiFe LDH on the Growth of Pt Nanoparticles and Hydrogen Evolution Performance
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.jpclett.1c02095 Yihan Feng 1, 2 , Ruguang Ma 1, 2 , Minmin Wang 3 , Jin Wang 3 , Tongming Sun 3 , Lanping Hu 3 , Jinli Zhu 3 , Yanfeng Tang 3 , Jiacheng Wang 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.jpclett.1c02095 Yihan Feng 1, 2 , Ruguang Ma 1, 2 , Minmin Wang 3 , Jin Wang 3 , Tongming Sun 3 , Lanping Hu 3 , Jinli Zhu 3 , Yanfeng Tang 3 , Jiacheng Wang 1, 2
Affiliation
|
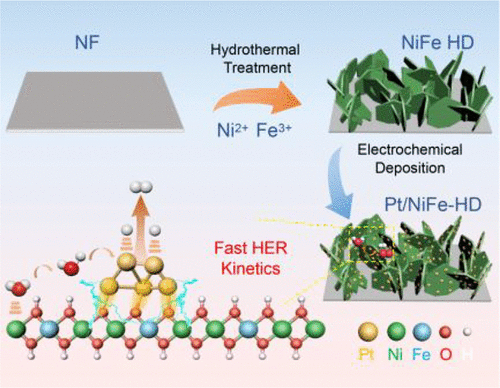
NiFe layered double hydroxides (LDHs) usually exhibit high water-dissociation ability in the alkaline media and also provide an ideal substrate for anchoring noble metals, such as platinum (Pt), due to the 2D microstructure. Appropriate regulation of the interaction between Pt and substrate could enhance the intrinsic activity of composite catalysts toward the hydrogen evolution reaction (HER) in the alkaline media. Herein, we electrodeposit Pt nanoparticles on amorphous NiFe LDH (Pt/NiFe-ED) or crystalline NiFe LDH (Pt/NiFe-HD) to regulate the interaction between Pt and NiFe LDH. Experimental results reveal that Pt nanoparticles on NiFe-ED are smaller than those on NiFe-HD and possess a narrower size distribution. Thus, Pt/NiFe-ED (300 μM) exhibits a much lower overpotential of 81 mV at 100 mA cm–2 than Pt/NiFe-HD. In contrast, Pt/NiFe-HD exhibits a higher intrinsic activity than Pt/NiFe-ED, which could be caused by the easily elongated Pt–O bond. These findings provide new opportunities to understand the relationship between activity and crystallinity of substrates in the composite electrocatalyst.
中文翻译:

NiFe LDH 结晶度对 Pt 纳米颗粒生长和析氢性能的影响
NiFe 层状双氢氧化物 (LDH) 在碱性介质中通常表现出很高的水解离能力,并且由于其二维微结构,还为锚定贵金属(如铂 (Pt))提供了理想的基材。适当调节铂与底物之间的相互作用可以提高复合催化剂在碱性介质中对析氢反应(HER)的固有活性。在此,我们在非晶 NiFe LDH (Pt/NiFe-ED) 或结晶 NiFe LDH (Pt/NiFe-HD) 上电沉积 Pt 纳米颗粒,以调节 Pt 和 NiFe LDH 之间的相互作用。实验结果表明,NiFe-ED 上的 Pt 纳米粒子比 NiFe-HD 上的 Pt 纳米粒子小,并且具有更窄的尺寸分布。因此,Pt/NiFe-ED (300 μM) 在 100 mA cm –2下表现出低得多的过电位 81 mV比 Pt/NiFe-HD。相比之下,Pt/NiFe-HD 表现出比 Pt/NiFe-ED 更高的内在活性,这可能是由容易拉长的 Pt-O 键引起的。这些发现为理解复合电催化剂中底物的活性与结晶度之间的关系提供了新的机会。
更新日期:2021-08-06
中文翻译:

NiFe LDH 结晶度对 Pt 纳米颗粒生长和析氢性能的影响
NiFe 层状双氢氧化物 (LDH) 在碱性介质中通常表现出很高的水解离能力,并且由于其二维微结构,还为锚定贵金属(如铂 (Pt))提供了理想的基材。适当调节铂与底物之间的相互作用可以提高复合催化剂在碱性介质中对析氢反应(HER)的固有活性。在此,我们在非晶 NiFe LDH (Pt/NiFe-ED) 或结晶 NiFe LDH (Pt/NiFe-HD) 上电沉积 Pt 纳米颗粒,以调节 Pt 和 NiFe LDH 之间的相互作用。实验结果表明,NiFe-ED 上的 Pt 纳米粒子比 NiFe-HD 上的 Pt 纳米粒子小,并且具有更窄的尺寸分布。因此,Pt/NiFe-ED (300 μM) 在 100 mA cm –2下表现出低得多的过电位 81 mV比 Pt/NiFe-HD。相比之下,Pt/NiFe-HD 表现出比 Pt/NiFe-ED 更高的内在活性,这可能是由容易拉长的 Pt-O 键引起的。这些发现为理解复合电催化剂中底物的活性与结晶度之间的关系提供了新的机会。































 京公网安备 11010802027423号
京公网安备 11010802027423号