Phytochemistry ( IF 3.2 ) Pub Date : 2021-07-27 , DOI: 10.1016/j.phytochem.2021.112889 Dan Thi Thuy Hang 1 , Do Thi Trang 1 , Duong Thi Dung 1 , Duong Thi Hai Yen 1 , Nguyen Huy Hoang 2 , Ngo Anh Bang 1 , Nguyen Thi Cuc 1 , Nguyen Xuan Nhiem 2 , Phan Thi Thanh Huong 1 , Bui Huu Tai 2 , Phan Van Kiem 2
|
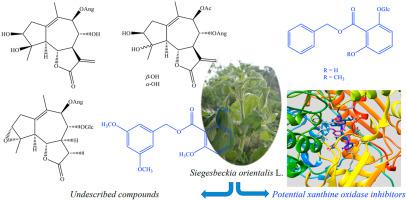
Five undescribed (four guaianolide sesquiterpenes and a benzoate ester derivative) and seven known compounds were isolated from the aerial parts of S. orientalis L. Their chemical structures were determined by extensive analysis of HR-ESI-MS and NMR spectroscopic methods. Absolute configurations were elucidated by experimental and TD-DFT calculated ECD spectra. Twelve isolated compounds exhibited potential xanthine oxidase inhibitory activity with IC50 values ranging from 0.76 ± 0.17 μM to 31.80 ± 0.97 μM. Molecular docking studies predicted that the binding energies of all isolated compounds with xanthine oxidase were lower than that of the positive control allopurinol. Benzyl 2-hydroxy-6-O-β-D-glucopyranosylbenzoate and benzyl 2-methoxy-6-O-β-D-glucopyranosylbenzoate displayed not only the best docking score but also the highest in vitro xanthine oxidase inhibitory activity with IC50 values of 0.76 ± 0.17 μM and 0.98 ± 0.26 μM, respectively.
中文翻译:

来自 Siegesbeckia orientalis L. 地上部分的愈创木内酯倍半萜烯和苯甲酸酯及其黄嘌呤氧化酶抑制活性
五个未描述的(4种guaianolide倍半萜烯和苯甲酸酯衍生物)和七个已知化合物从地上部分分离S.侧柏它们的化学结构通过HR-ESI-MS和NMR光谱法的广泛的分析来确定L.。通过实验和 TD-DFT 计算的 ECD 光谱阐明了绝对构型。12 种分离的化合物表现出潜在的黄嘌呤氧化酶抑制活性,IC 50值范围从 0.76 ± 0.17 μM 到 31.80 ± 0.97 μM。分子对接研究预测,所有分离的化合物与黄嘌呤氧化酶的结合能低于阳性对照别嘌呤醇的结合能。苄基2-羟基-6- O - β-D-吡喃葡萄糖基苯甲酸酯和苄基 2-甲氧基-6- O - β -D-吡喃葡萄糖基苯甲酸酯不仅显示出最佳对接评分,而且体外黄嘌呤氧化酶抑制活性最高,IC 50值为 0.76 ± 0.17 μM 和 0.98 ± 0.26 μM , 分别。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号