Cell Reports ( IF 7.5 ) Pub Date : 2021-07-27 , DOI: 10.1016/j.celrep.2021.109455 Francesca De Bacco 1 , Francesca Orzan 1 , Jessica Erriquez 2 , Elena Casanova 1 , Ludovic Barault 3 , Raffaella Albano 2 , Antonio D'Ambrosio 1 , Viola Bigatto 1 , Gigliola Reato 1 , Monica Patanè 4 , Bianca Pollo 4 , Geoffrey Kuesters 5 , Carmine Dell'Aglio 6 , Laura Casorzo 7 , Serena Pellegatta 4 , Gaetano Finocchiaro 8 , Paolo M Comoglio 9 , Carla Boccaccio 10
|
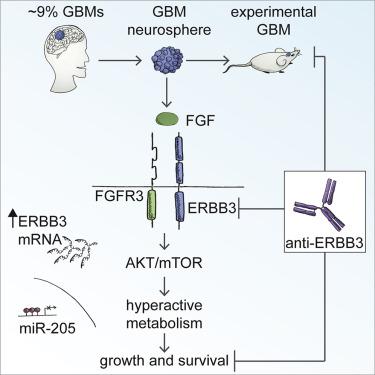
In glioblastoma (GBM), the most frequent and lethal brain tumor, therapies suppressing recurrently altered signaling pathways failed to extend survival. However, in patient subsets, specific genetic lesions can confer sensitivity to targeted agents. By exploiting an integrated model based on patient-derived stem-like cells, faithfully recapitulating the original GBMs in vitro and in vivo, here, we identify a human GBM subset (∼9% of all GBMs) characterized by ERBB3 overexpression and nuclear accumulation. ERBB3 overexpression is driven by inheritable promoter methylation or post-transcriptional silencing of the oncosuppressor miR-205 and sustains the malignant phenotype. Overexpressed ERBB3 behaves as a specific signaling platform for fibroblast growth factor receptor (FGFR), driving PI3K/AKT/mTOR pathway hyperactivation, and overall metabolic upregulation. As a result, ERBB3 inhibition by specific antibodies is lethal for GBM stem-like cells and xenotransplants. These findings highlight a subset of patients eligible for ERBB3-targeted therapy.
中文翻译:

由于 miR-205 失活导致的 ERBB3 过表达赋予胶质母细胞瘤中对 FGF、代谢激活和 ERBB3 靶向的敏感性
在胶质母细胞瘤 (GBM) 中,最常见和最致命的脑肿瘤,抑制反复改变的信号通路的疗法未能延长生存期。然而,在患者亚群中,特定的遗传病变可以赋予对靶向药物的敏感性。通过利用基于患者来源的干细胞样细胞的综合模型,在体外和体内忠实再现原始 GBM,在这里,我们确定了一个以 ERBB3 过表达和核积累为特征的人类 GBM 子集(约占所有 GBM 的 9%)。ERBB3 过表达由可遗传的启动子甲基化或抑癌基因 miR-205 的转录后沉默驱动,并维持恶性表型。过表达的 ERBB3 充当成纤维细胞生长因子受体 (FGFR) 的特定信号平台,驱动 PI3K/AKT/mTOR 通路过度激活和整体代谢上调。因此,特异性抗体对 ERBB3 的抑制对 GBM 干细胞样细胞和异种移植是致命的。这些发现突出了一部分符合 ERBB3 靶向治疗的患者。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号