当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of the plant receptor-like kinase TDR in complex with the TDIF peptide.
Nature Communications ( IF 14.7 ) Pub Date : 2016-08-08 , DOI: 10.1038/ncomms12383 Junko Morita , Kazuki Kato , Takanori Nakane , Yuki Kondo , Hiroo Fukuda , Hiroshi Nishimasu , Ryuichiro Ishitani , Osamu Nureki
Nature Communications ( IF 14.7 ) Pub Date : 2016-08-08 , DOI: 10.1038/ncomms12383 Junko Morita , Kazuki Kato , Takanori Nakane , Yuki Kondo , Hiroo Fukuda , Hiroshi Nishimasu , Ryuichiro Ishitani , Osamu Nureki
|
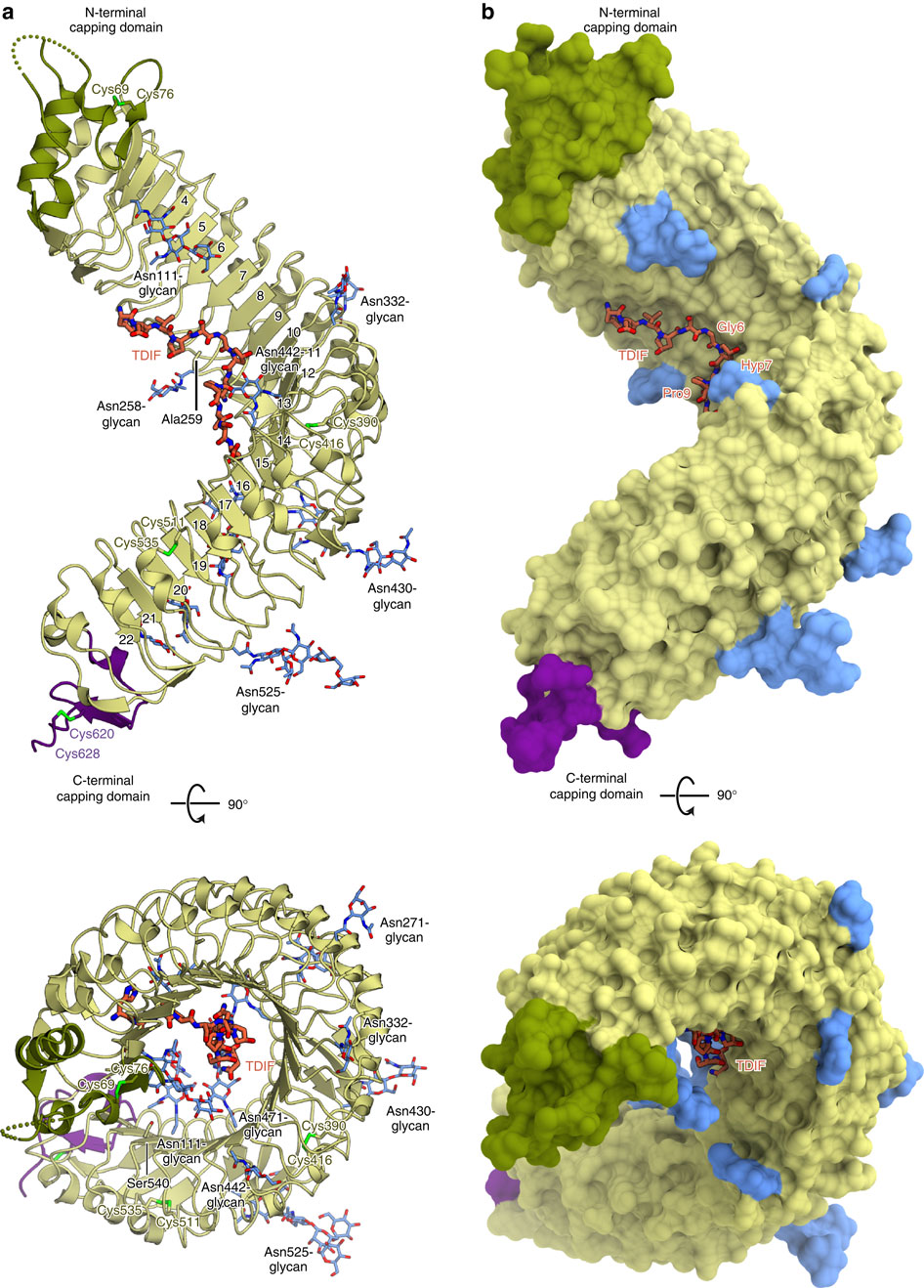
In plants, leucine-rich repeat receptor-like kinases (LRR-RKs) perceive ligands, including peptides and small molecules, to regulate various physiological processes. TDIF, a member of the CLE peptide family, specifically interacts with the LRR-RK TDR to inhibit meristem differentiation into tracheary elements, and promotes cell proliferation. Here we report the crystal structure of the extracellular domain of TDR in complex with the TDIF peptide. The extracellular domain of TDR adopts a superhelical structure comprising 22 LRRs, and specifically recognizes TDIF by its inner concave surface. Together with our biochemical and sequence analyses, our structure reveals a conserved TDIF-recognition mechanism of TDR among plant species. Furthermore, a structural comparison of TDR with other plant LRR-RKs suggested the activation mechanism of TDR by TDIF. The structure of this CLE peptide receptor provides insights into the recognition mechanism of the CLE family peptides.
中文翻译:

与TDIF肽复合的植物受体样激酶TDR的晶体结构。
在植物中,富含亮氨酸的重复受体样激酶(LRR-RKs)感知配体,包括肽和小分子,以调节各种生理过程。TDIF是CLE肽家族的成员,与LRR-RK TDR特异性相互作用,抑制分生组织分化为气管元件,并促进细胞增殖。在这里,我们报告与TDIF肽复合的TDR胞外域的晶体结构。TDR的胞外域采用包含22个LRR的超螺旋结构,并通过其内凹表面特异性识别TDIF。连同我们的生化和序列分析,我们的结构揭示了植物物种中TDR的保守TDIF识别机制。此外,TDR与其他植物LRR-RK的结构比较表明TDIF可以激活TDR。
更新日期:2016-08-11
中文翻译:

与TDIF肽复合的植物受体样激酶TDR的晶体结构。
在植物中,富含亮氨酸的重复受体样激酶(LRR-RKs)感知配体,包括肽和小分子,以调节各种生理过程。TDIF是CLE肽家族的成员,与LRR-RK TDR特异性相互作用,抑制分生组织分化为气管元件,并促进细胞增殖。在这里,我们报告与TDIF肽复合的TDR胞外域的晶体结构。TDR的胞外域采用包含22个LRR的超螺旋结构,并通过其内凹表面特异性识别TDIF。连同我们的生化和序列分析,我们的结构揭示了植物物种中TDR的保守TDIF识别机制。此外,TDR与其他植物LRR-RK的结构比较表明TDIF可以激活TDR。






























 京公网安备 11010802027423号
京公网安备 11010802027423号