Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-07-26 , DOI: 10.1016/j.bioorg.2021.105210 Lalitha Gummidi 1 , Nagaraju Kerru 1 , Oluwakemi Ebenezer 1 , Paul Awolade 1 , Olakunle Sanni 2 , Md Shahidul Islam 2 , Parvesh Singh 1
|
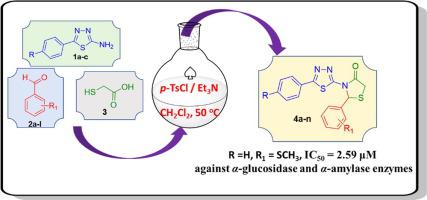
A simple and efficient protocol was developed to synthesize a new library of thiazolidine-4-one molecular hybrids (4a-n) via a one-pot multicomponent reaction involving 5-substituted phenyl-1,3,4-thiadiazol-2-amines, substituted benzaldehydes and 2-mercaptoacetic acid. The synthesized compounds were evaluated in vitro for their antidiabetic activities through α-glucosidase and α-amylase inhibition as well as their antioxidant and antimicrobial potentials. Compound 4e exhibited the most promising α-glucosidase and α-amylase inhibition with an IC50 value of 2.59 μM, which is ~1.5- and 14-fold superior as compared to the standard inhibitor acarbose. Structure-activity relationship (SAR) analysis revealed that the nature and position of substituents on the phenyl rings had a significant effect on the inhibitory potency.
中文翻译:

通过α-葡萄糖苷酶和α-淀粉酶抑制合成新的1,3,4-噻二唑-噻唑烷-4-one分子杂化物作为有前景的抗糖尿病药物的多组分反应
开发了一种简单有效的方案,通过涉及 5-取代苯基-1,3,4-噻二唑-2-胺的一锅多组分反应合成新的噻唑烷-4-one 分子杂化物 ( 4a-n )库,取代的苯甲醛和 2-巯基乙酸。通过抑制α-葡萄糖苷酶和α-淀粉酶以及它们的抗氧化和抗菌潜力,体外评估合成的化合物的抗糖尿病活性。化合物4e表现出最有希望的α-葡萄糖苷酶和α-淀粉酶抑制作用,IC 50值为 2.59 μM,与标准抑制剂阿卡波糖相比,分别高出约 1.5 倍和 14 倍。构效关系(SAR)分析表明,苯环上取代基的性质和位置对抑制效力有显着影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号