Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Activity of Ethoxylate Surfactants with Different Hydrophobic Architectures: The Effect of Layer Substructure on Surface Tension and Adsorption
Langmuir ( IF 3.7 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.langmuir.1c01588 James R Reeve 1 , Robert K Thomas 1 , Jeffrey Penfold 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2021-07-26 , DOI: 10.1021/acs.langmuir.1c01588 James R Reeve 1 , Robert K Thomas 1 , Jeffrey Penfold 1, 2
Affiliation
|
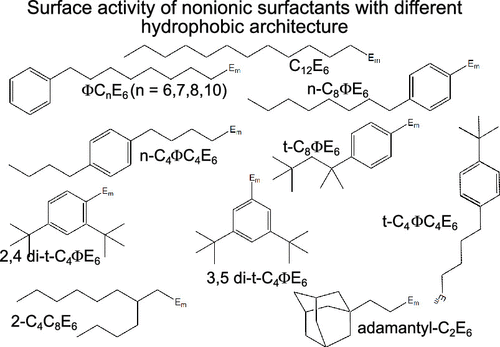
A series of nonionic ethoxylate surfactants containing different combinations of alkyl, phenyl, and adamantyl units in nine different arrangements, each combined with penta- and hexa-ethylene glycol groups, were synthesized and purified. The surface properties of all of the surfactants were investigated at the air–water (A–W) interface using surface tension (ST) to determine the limiting surface excess (Γlim), the limiting surface tension (σlim), and the critical micelle concentration (CMC). A smaller selection was investigated at the hydrophilic silica–water interface by neutron reflectometry to obtain the thickness of the adsorbed layer and the total adsorption at the CMC. An unusual and largely unrecognized feature of the ethoxylate group is that it is both hydrophilic and hydrophobic. It was found possible to account for the variation of σlim and Γlim of all of the adsorbed layers in terms of a balance of the estimated STs of the sublayers forming the overall adsorbed layer, including that of the underlying ethoxylate layer. The values of σlim were found to be highest for phenyl- and adamantyl-capped surfactants and lowest mainly when there was more than one methyl group at the surface. However, in terms of the concentration required to reach a given low ST, increasing the number of attached methyl groups was found to be less effective than using a smaller number of better-placed methyl groups. At the solid–liquid interface, adsorption at or above the CMC was in all cases in the form of a fragmented bilayer whose coverage varied approximately linearly with the packing parameter. However, results on the phenyl-capped surfactants showed that the high ST exhibited by these surfactants at the A–W interface becomes a high cohesion energy in the interior of the bilayer and they exhibited significantly higher adsorption than expected from simple packing arguments.
中文翻译:

具有不同疏水结构的乙氧基化物表面活性剂的表面活性:层亚结构对表面张力和吸附的影响
合成并纯化了一系列非离子乙氧基化物表面活性剂,这些表面活性剂包含九种不同排列的烷基、苯基和金刚烷基单元的不同组合,每种都与五和六乙二醇基团结合。使用表面张力 (ST) 在空气-水 (A-W) 界面研究所有表面活性剂的表面性质,以确定极限表面过量 (Γ lim )、极限表面张力 (σ lim) 和临界胶束浓度 (CMC)。通过中子反射法在亲水性二氧化硅 - 水界面研究了较小的选择,以获得吸附层的厚度和 CMC 的总吸附量。乙氧基化物基团的一个不寻常且在很大程度上未被认识到的特征是它既亲水又疏水。发现可以根据形成整个吸附层的子层的估计 ST 的平衡来解释所有吸附层的 σ lim和 Γ lim的变化,包括下面的乙氧基化物层。σ lim的值发现苯基和金刚烷基封端的表面活性剂最高,而最低的主要是当表面有一个以上的甲基时。然而,就达到给定低 ST 所需的浓度而言,发现增加连接甲基的数量不如使用较少数量的更好放置的甲基有效。在固-液界面,CMC 处或上方的吸附在所有情况下都以碎片双层的形式存在,其覆盖率随填充参数近似线性变化。然而,苯基封端的表面活性剂的结果表明,这些表面活性剂在 A-W 界面表现出的高 ST 变成了双层内部的高内聚能,并且它们表现出比简单堆积参数预期的明显更高的吸附。
更新日期:2021-08-03
中文翻译:

具有不同疏水结构的乙氧基化物表面活性剂的表面活性:层亚结构对表面张力和吸附的影响
合成并纯化了一系列非离子乙氧基化物表面活性剂,这些表面活性剂包含九种不同排列的烷基、苯基和金刚烷基单元的不同组合,每种都与五和六乙二醇基团结合。使用表面张力 (ST) 在空气-水 (A-W) 界面研究所有表面活性剂的表面性质,以确定极限表面过量 (Γ lim )、极限表面张力 (σ lim) 和临界胶束浓度 (CMC)。通过中子反射法在亲水性二氧化硅 - 水界面研究了较小的选择,以获得吸附层的厚度和 CMC 的总吸附量。乙氧基化物基团的一个不寻常且在很大程度上未被认识到的特征是它既亲水又疏水。发现可以根据形成整个吸附层的子层的估计 ST 的平衡来解释所有吸附层的 σ lim和 Γ lim的变化,包括下面的乙氧基化物层。σ lim的值发现苯基和金刚烷基封端的表面活性剂最高,而最低的主要是当表面有一个以上的甲基时。然而,就达到给定低 ST 所需的浓度而言,发现增加连接甲基的数量不如使用较少数量的更好放置的甲基有效。在固-液界面,CMC 处或上方的吸附在所有情况下都以碎片双层的形式存在,其覆盖率随填充参数近似线性变化。然而,苯基封端的表面活性剂的结果表明,这些表面活性剂在 A-W 界面表现出的高 ST 变成了双层内部的高内聚能,并且它们表现出比简单堆积参数预期的明显更高的吸附。






























 京公网安备 11010802027423号
京公网安备 11010802027423号