当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide Cyclization Mediated by Metal-Free S-Arylation: S-Protected Cysteine Sulfoxide as an Umpolung of the Cysteine Nucleophile
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-07-23 , DOI: 10.1002/chem.202102420 Daishiro Kobayashi 1 , Yutaka Kohmura 1 , Toshihiko Sugiki 2 , Eisuke Kuraoka 1 , Masaya Denda 1 , Toshimichi Fujiwara 2 , Akira Otaka 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2021-07-23 , DOI: 10.1002/chem.202102420 Daishiro Kobayashi 1 , Yutaka Kohmura 1 , Toshihiko Sugiki 2 , Eisuke Kuraoka 1 , Masaya Denda 1 , Toshimichi Fujiwara 2 , Akira Otaka 1
Affiliation
|
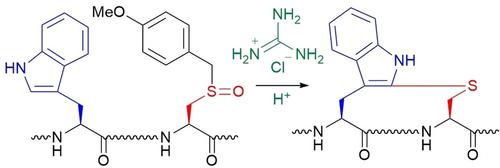
Covalent linking of side chains provides a method to produce cyclic or stapled peptides that are important in developing peptide-based drugs. A variety of crosslinking formats contribute to fixing the active conformer and prolonging its biological activity under physiological conditions. One format uses the cysteine thiol to participate in crosslinking through nucleophilic thiolate anions or thiyl radicals to form thioether and disulfide bonds. Removal of the S-protection from an S-protected Cys derivative generates the thiol, which functions as a nucleophile. S-Oxidation of a protected Cys allows the formation of a sulfoxide that operates as an umpolung electrophile. Herein, the applicability of S-p-methoxybenzyl Cys sulfoxide (Cys(MBzl)(O)) to the formation of a thioether linkage between tryptophan and Cys has been investigated. The reaction of peptides containing Cys(MBzl)(O) and Trp with trifluoromethanesulfonic acid (TFMSA) or methanesulfonic acid (MSA) in TFA in the presence of guanidine hydrochloride (Gn ⋅ HCl) proceeded to give cyclic or stapled peptides possessing the Cys-Trp thioether linkage. In this reaction, strong acids such as TFMSA or MSA are necessary to activate the sulfoxide. Additionally, Gn ⋅ HCl plays a critical role in producing an electrophilic Cys derivative that combines with the indole by aromatic electrophilic substitution. The findings led us to conclude that the less-electrophilic Cys(MBzl)(O) serves as an acid-activated umpolung of a Cys nucleophile and is useful for S-arylation-mediated peptide cyclization.
中文翻译:

由无金属 S-芳基化介导的肽环化:S-保护的半胱氨酸亚砜作为半胱氨酸亲核试剂的 Umpolung
侧链的共价连接提供了一种生产环状或订书钉肽的方法,这对于开发基于肽的药物很重要。多种交联形式有助于固定活性构象异构体并延长其在生理条件下的生物活性。一种形式使用半胱氨酸硫醇通过亲核硫醇阴离子或硫基自由基参与交联以形成硫醚和二硫键。从 S-保护的 Cys 衍生物上去除 S-保护会产生硫醇,它起到亲核试剂的作用。受保护的 Cys 的 S-氧化允许形成亚砜,作为 umpolung 亲电试剂。在此,S- p的适用性已经研究了 -甲氧基苄基半胱氨酸亚砜 (Cys(MBzl)(O)) 在色氨酸和半胱氨酸之间形成硫醚键。在盐酸胍 (Gn·HCl) 存在下,含有 Cys(MBzl)(O) 和 Trp 的肽与三氟甲磺酸 (TFMSA) 或甲磺酸 (MSA) 在 TFA 中的反应进行得到具有 Cys- Trp 硫醚键。在这个反应中,强酸如 TFMSA 或 MSA 是激活亚砜所必需的。此外,Gn ⋅ HCl 在通过芳香亲电取代与吲哚结合的亲电 Cys 衍生物中起着关键作用。研究结果使我们得出结论,亲电性较低的 Cys(MBzl)(O) 作为 Cys 亲核试剂的酸活化 umpolung,可用于 S-芳基化介导的肽环化。
更新日期:2021-07-23
中文翻译:

由无金属 S-芳基化介导的肽环化:S-保护的半胱氨酸亚砜作为半胱氨酸亲核试剂的 Umpolung
侧链的共价连接提供了一种生产环状或订书钉肽的方法,这对于开发基于肽的药物很重要。多种交联形式有助于固定活性构象异构体并延长其在生理条件下的生物活性。一种形式使用半胱氨酸硫醇通过亲核硫醇阴离子或硫基自由基参与交联以形成硫醚和二硫键。从 S-保护的 Cys 衍生物上去除 S-保护会产生硫醇,它起到亲核试剂的作用。受保护的 Cys 的 S-氧化允许形成亚砜,作为 umpolung 亲电试剂。在此,S- p的适用性已经研究了 -甲氧基苄基半胱氨酸亚砜 (Cys(MBzl)(O)) 在色氨酸和半胱氨酸之间形成硫醚键。在盐酸胍 (Gn·HCl) 存在下,含有 Cys(MBzl)(O) 和 Trp 的肽与三氟甲磺酸 (TFMSA) 或甲磺酸 (MSA) 在 TFA 中的反应进行得到具有 Cys- Trp 硫醚键。在这个反应中,强酸如 TFMSA 或 MSA 是激活亚砜所必需的。此外,Gn ⋅ HCl 在通过芳香亲电取代与吲哚结合的亲电 Cys 衍生物中起着关键作用。研究结果使我们得出结论,亲电性较低的 Cys(MBzl)(O) 作为 Cys 亲核试剂的酸活化 umpolung,可用于 S-芳基化介导的肽环化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号