Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-07-24 , DOI: 10.1016/j.bioorg.2021.105205 Menna A Ewida 1 , Heba A Ewida 2 , Mahmoud S Ahmed 3 , Heba Abdelrasheed Allam 4 , Ramzia I ElBagary 1 , Riham F George 4 , Hanan H Georgey 5 , Hussein I El-Subbagh 6
|
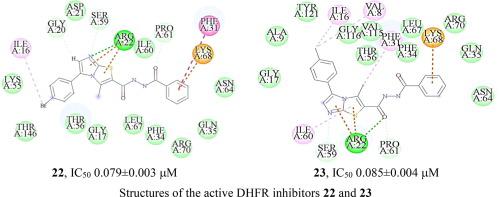
Inhibiting the Dihydrofolate reductase (DHFR) enzyme has been validated in multiple clinical manifestations related to bacterial infection, malaria, and multiple types of cancer. Herein, novel series of 3-methyl-imidazo[2,1-b] thiazole-based analogs were synthesized and biologically evaluated for their in vitro inhibitory profile towards DHFR. Compounds 22 and 23 exhibited potent inhibitory profile targeting DHFR (IC50 0.079 and 0.085 µM, respectively comparable to MTX IC50 0.087 µM). Compounds 22 and 23 showed promising cytotoxicity against MCF7 breast cancer cell lines inducing cell cycle arrest and apoptosis. Furthermore, Compound 23 showed its potential to reduce body weight and tumor volume significantly, using Ehrlich ascites carcinoma (EAC) solid tumor animal model of breast cancer, compared to control-treated groups. Further, molecular modeling simulations validated the potential of 22 and 23 to have high affinity binding towards Arg22 and Phe31 residues via π-π interaction and hydrogen bonding within DHFR binding pocket. Computer-assisted ADMET study suggested that the newly synthesized analogs could have high penetration to the blood brain barrier (BBB), better intestinal absorption, non-inhibitors of CYP2D6, adequate plasma protein binding and good passive oral absorption. The obtained model and pattern of substitution could be used for further development of DHFR inhibitors.
中文翻译:

3-甲基-咪唑并[2,1-b]噻唑衍生物作为一类新的抗叶酸剂:合成、体外/体内生物评价和分子建模模拟
抑制二氢叶酸还原酶 (DHFR) 已在与细菌感染、疟疾和多种癌症相关的多种临床表现中得到验证。在此,合成了新系列的 3-甲基-咪唑并[2,1- b ] 噻唑类类似物,并对其在体外对 DHFR 的抑制特性进行了生物学评估。化合物22和23表现出针对 DHFR 的有效抑制特性(IC 50 0.079 和 0.085 µM,分别与 MTX IC 50 0.087 µM相当)。化合物22和23对诱导细胞周期停滞和凋亡的 MCF7 乳腺癌细胞系显示出有希望的细胞毒性。此外,与对照组相比,化合物23使用乳腺癌的艾氏腹水癌 (EAC) 实体瘤动物模型显示其显着降低体重和肿瘤体积的潜力。此外,分子建模模拟验证了22和23通过以下方式与 Arg22 和 Phe31 残基具有高亲和力结合的潜力DHFR 结合口袋内的 π-π 相互作用和氢键。计算机辅助的 ADMET 研究表明,新合成的类似物对血脑屏障 (BBB) 的渗透率高、肠道吸收更好、CYP2D6 非抑制剂、足够的血浆蛋白结合和良好的被动口服吸收。获得的模型和取代模式可用于进一步开发 DHFR 抑制剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号