当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Biological Evaluation of Kibdelone C and Its Simplified Derivatives
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-09 , DOI: 10.1021/jacs.6b05484
Janjira Rujirawanich 1 , Soyeon Kim 1 , Ai-Jun Ma 1 , John R. Butler 1 , Yizhong Wang 1 , Chao Wang 1 , Michael Rosen 1 , Bruce Posner 1 , Deepak Nijhawan 1 , Joseph M. Ready 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-09 , DOI: 10.1021/jacs.6b05484
Janjira Rujirawanich 1 , Soyeon Kim 1 , Ai-Jun Ma 1 , John R. Butler 1 , Yizhong Wang 1 , Chao Wang 1 , Michael Rosen 1 , Bruce Posner 1 , Deepak Nijhawan 1 , Joseph M. Ready 1
Affiliation
|
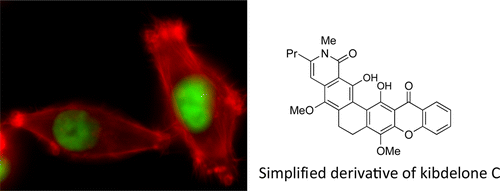
Poylcyclic tetrahydroxanthones comprise a large class of cytototoxic natural products. No mechanism of action has been described for any member of the family. We report the synthesis of kibdelone C and several simplified analogs. Both enantiomers of kibdeleone C show low nanomolar cytotoxicity toward multiple human cancer cell lines. Moreover, several simplified derivatives with improved chemical stability display higher activity than the natural product itself. In vitro studies rule out interaction with DNA or inhibition of topoisomerase, both of which are common modes of action for polycyclic aromatic compounds. However, celluar studies reveal that kibdelone C and its simplified derivatives disrupt the actin cytoseketon without directly binding actin or affecting its polymerization in vitro.
中文翻译:

Kibdelone C 及其简化衍生物的合成和生物学评价
多环四氢氧杂蒽酮包括一大类细胞毒性天然产物。没有描述任何家族成员的作用机制。我们报告了 kibdelone C 和几种简化类似物的合成。kibdeleone C 的两种对映异构体对多种人类癌细胞系都显示出低纳摩尔的细胞毒性。此外,一些具有改进化学稳定性的简化衍生物显示出比天然产物本身更高的活性。体外研究排除了与 DNA 的相互作用或拓扑异构酶的抑制,这两者都是多环芳族化合物的常见作用方式。然而,细胞研究表明,kibdelone C 及其简化的衍生物会破坏肌动蛋白 cytoseketon,而不直接结合肌动蛋白或影响其体外聚合。
更新日期:2016-08-09
中文翻译:

Kibdelone C 及其简化衍生物的合成和生物学评价
多环四氢氧杂蒽酮包括一大类细胞毒性天然产物。没有描述任何家族成员的作用机制。我们报告了 kibdelone C 和几种简化类似物的合成。kibdeleone C 的两种对映异构体对多种人类癌细胞系都显示出低纳摩尔的细胞毒性。此外,一些具有改进化学稳定性的简化衍生物显示出比天然产物本身更高的活性。体外研究排除了与 DNA 的相互作用或拓扑异构酶的抑制,这两者都是多环芳族化合物的常见作用方式。然而,细胞研究表明,kibdelone C 及其简化的衍生物会破坏肌动蛋白 cytoseketon,而不直接结合肌动蛋白或影响其体外聚合。

































 京公网安备 11010802027423号
京公网安备 11010802027423号