当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypoxia-Responsive Gene Editing to Reduce Tumor Thermal Tolerance for Mild-Photothermal Therapy
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-23 , DOI: 10.1002/anie.202107036 Xueqing Li 1 , Yongchun Pan 1 , Chao Chen 2 , Yanfeng Gao 1 , Xinli Liu 1 , Kaiyong Yang 2 , Xiaowei Luan 1 , Dongtao Zhou 1 , Fei Zeng 1 , Xin Han 2 , Yujun Song 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-23 , DOI: 10.1002/anie.202107036 Xueqing Li 1 , Yongchun Pan 1 , Chao Chen 2 , Yanfeng Gao 1 , Xinli Liu 1 , Kaiyong Yang 2 , Xiaowei Luan 1 , Dongtao Zhou 1 , Fei Zeng 1 , Xin Han 2 , Yujun Song 1
Affiliation
|
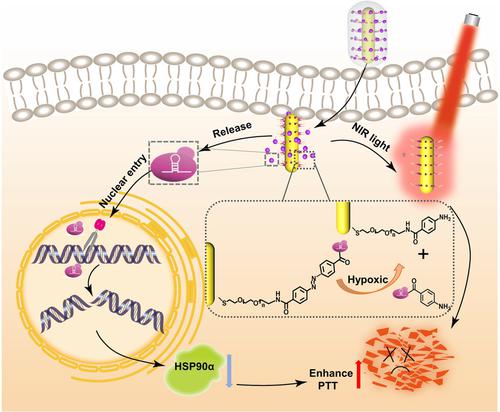
Near-infrared (NIR)-light-triggered photothermal therapy (PTT) is usually associated with undesirable damage to healthy organs nearby due to the high temperatures (>50 °C) available for tumor ablation. Low-temperature PTT would therefore have tremendous value for clinical application. Here, we construct a hypoxia-responsive gold nanorods (AuNRs)-based nanocomposite of CRISPR-Cas9 for mild-photothermal therapy via tumor-targeted gene editing. AuNRs are modified with azobenzene-4,4′-dicarboxylic acid (p-AZO) to achieve on-demand release of CRISPR-Cas9 using hypoxia-responsive azo bonds. In the hypoxic tumor microenvironment, the azo groups of the hypoxia-activated CRISPR-Cas9 nanosystem based on gold nanorods (APACPs) are selectively reduced by the overexpression of reductases, leading to the release of Cas9 and subsequent gene editing. Owing to the knockout of HSP90α for reducing the thermal resistance of cancer cells, highly effective tumor ablation both in vitro and in vivo was achieved with APACPs under mild PTT.
中文翻译:

低氧反应性基因编辑可降低温和光热疗法的肿瘤热耐受性
由于可用于肿瘤消融的高温 (>50 °C),近红外 (NIR) 光触发光热疗法 (PTT) 通常会对附近的健康器官造成不良损害。因此低温PTT对临床应用具有巨大的价值。在这里,我们构建了一种基于缺氧响应金纳米棒 (AuNRs) 的 CRISPR-Cas9 纳米复合材料,用于通过肿瘤靶向基因编辑进行温和的光热治疗。AuNRs 用偶氮苯-4,4'-二羧酸(p-AZO) 使用缺氧响应偶氮键实现 CRISPR-Cas9 的按需释放。在缺氧的肿瘤微环境中,基于金纳米棒(APACPs)的缺氧激活的 CRISPR-Cas9 纳米系统的偶氮基团被还原酶的过表达选择性还原,导致 Cas9 的释放和随后的基因编辑。由于敲除 HSP90α 以降低癌细胞的热阻,在温和的 PTT 下使用 APACP 实现了高效的体外和体内肿瘤消融。
更新日期:2021-09-14
中文翻译:

低氧反应性基因编辑可降低温和光热疗法的肿瘤热耐受性
由于可用于肿瘤消融的高温 (>50 °C),近红外 (NIR) 光触发光热疗法 (PTT) 通常会对附近的健康器官造成不良损害。因此低温PTT对临床应用具有巨大的价值。在这里,我们构建了一种基于缺氧响应金纳米棒 (AuNRs) 的 CRISPR-Cas9 纳米复合材料,用于通过肿瘤靶向基因编辑进行温和的光热治疗。AuNRs 用偶氮苯-4,4'-二羧酸(p-AZO) 使用缺氧响应偶氮键实现 CRISPR-Cas9 的按需释放。在缺氧的肿瘤微环境中,基于金纳米棒(APACPs)的缺氧激活的 CRISPR-Cas9 纳米系统的偶氮基团被还原酶的过表达选择性还原,导致 Cas9 的释放和随后的基因编辑。由于敲除 HSP90α 以降低癌细胞的热阻,在温和的 PTT 下使用 APACP 实现了高效的体外和体内肿瘤消融。






























 京公网安备 11010802027423号
京公网安备 11010802027423号