当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different Etching Mechanisms of Diamond by Oxygen and Hydrogen Plasma: a Reactive Molecular Dynamics Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-07-21 , DOI: 10.1021/acs.jpcc.1c03919 Jingxiang Xu 1, 2, 3 , Kang Lu 1 , Ding Fan 4 , Yang Wang 2, 5 , Shaolin Xu 6 , Momoji Kubo 2, 7
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-07-21 , DOI: 10.1021/acs.jpcc.1c03919 Jingxiang Xu 1, 2, 3 , Kang Lu 1 , Ding Fan 4 , Yang Wang 2, 5 , Shaolin Xu 6 , Momoji Kubo 2, 7
Affiliation
|
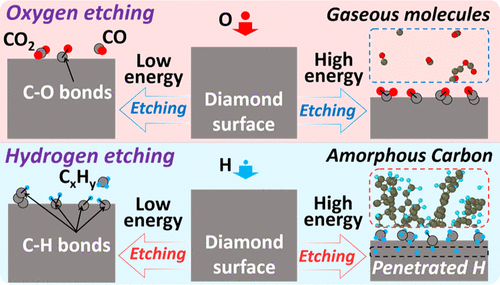
Understanding the plasma etching mechanism of diamond is of great significance to promote diamond applications; however, insights into the atomic-scale etching mechanisms are hidden by the complex chemical reactions during the etching process due to the lack of an in situ characterization technique into the etching process. Herein, we conducted an etching simulation of diamond using the reactive molecular dynamics simulation method to comparatively investigate the different etching mechanisms of diamond by oxygen and hydrogen plasma with different incident energies. In the case of oxygen etching, at all tested incident energies, C–C bonds on the diamond surface are dissociated by the irradiated oxygen, and carbon atoms of diamond are etched away via the generation and desorption of gaseous carbon monoxide and carbon dioxide molecules. In the case of hydrogen etching, at low incident energies, we revealed that the carbon atoms are etched through the desorption of gaseous hydrocarbon molecules similar to the oxygen etching mechanism, while with increasing the incident energies, we interestingly observe an obvious different etching mechanism, that is, the irradiated hydrogen penetrates into the inside of diamond resulting in the formation of the hydrogenated amorphous layer which is then exfoliated from the diamond surface. Meanwhile, we revealed that the loss rate of carbon atoms in the diamond structure by oxygen is higher at low incident energies but lower at high incident energies than that by hydrogen. This study provides more insights into the etching mechanism of oxygen and hydrogen plasma and offers useful theoretical guidance for designing and controlling the etching process.
中文翻译:

氧和氢等离子体对金刚石的不同蚀刻机制:反应性分子动力学研究
了解金刚石的等离子刻蚀机理对促进金刚石应用具有重要意义;然而,由于缺乏原位蚀刻,蚀刻过程中复杂的化学反应掩盖了对原子级蚀刻机制的深入了解。刻蚀工艺中的表征技术。在此,我们利用反应性分子动力学模拟方法对金刚石进行了刻蚀模拟,以比较研究不同入射能量的氧和氢等离子体对金刚石的不同刻蚀机理。在氧气蚀刻的情况下,在所有测试的入射能量下,金刚石表面的 C-C 键被辐照的氧气解离,金刚石的碳原子通过气态一氧化碳和二氧化碳分子的产生和解吸而被蚀刻掉。在氢蚀刻的情况下,在低入射能量下,我们发现碳原子通过类似于氧蚀刻机制的气态烃分子的解吸被蚀刻,同时随着入射能量的增加,我们有趣地观察到一个明显不同的蚀刻机制,即被照射的氢渗透到金刚石内部,导致形成氢化非晶层,然后从金刚石表面剥离。同时,我们发现氧对金刚石结构中碳原子的损失率在低入射能时更高,但在高入射能时比氢低。该研究为氧和氢等离子体的蚀刻机制提供了更多见解,并为设计和控制蚀刻工艺提供了有用的理论指导。我们发现氧在金刚石结构中的碳原子损失率在低入射能时更高,但在高入射能时比氢低。该研究为氧和氢等离子体的蚀刻机制提供了更多见解,并为设计和控制蚀刻工艺提供了有用的理论指导。我们发现氧在金刚石结构中的碳原子损失率在低入射能时更高,但在高入射能时比氢低。该研究为氧和氢等离子体的蚀刻机制提供了更多见解,并为设计和控制蚀刻工艺提供了有用的理论指导。
更新日期:2021-08-06
中文翻译:

氧和氢等离子体对金刚石的不同蚀刻机制:反应性分子动力学研究
了解金刚石的等离子刻蚀机理对促进金刚石应用具有重要意义;然而,由于缺乏原位蚀刻,蚀刻过程中复杂的化学反应掩盖了对原子级蚀刻机制的深入了解。刻蚀工艺中的表征技术。在此,我们利用反应性分子动力学模拟方法对金刚石进行了刻蚀模拟,以比较研究不同入射能量的氧和氢等离子体对金刚石的不同刻蚀机理。在氧气蚀刻的情况下,在所有测试的入射能量下,金刚石表面的 C-C 键被辐照的氧气解离,金刚石的碳原子通过气态一氧化碳和二氧化碳分子的产生和解吸而被蚀刻掉。在氢蚀刻的情况下,在低入射能量下,我们发现碳原子通过类似于氧蚀刻机制的气态烃分子的解吸被蚀刻,同时随着入射能量的增加,我们有趣地观察到一个明显不同的蚀刻机制,即被照射的氢渗透到金刚石内部,导致形成氢化非晶层,然后从金刚石表面剥离。同时,我们发现氧对金刚石结构中碳原子的损失率在低入射能时更高,但在高入射能时比氢低。该研究为氧和氢等离子体的蚀刻机制提供了更多见解,并为设计和控制蚀刻工艺提供了有用的理论指导。我们发现氧在金刚石结构中的碳原子损失率在低入射能时更高,但在高入射能时比氢低。该研究为氧和氢等离子体的蚀刻机制提供了更多见解,并为设计和控制蚀刻工艺提供了有用的理论指导。我们发现氧在金刚石结构中的碳原子损失率在低入射能时更高,但在高入射能时比氢低。该研究为氧和氢等离子体的蚀刻机制提供了更多见解,并为设计和控制蚀刻工艺提供了有用的理论指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号