当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Doped Nickel Phosphate as Synergistic Electrocatalyst for Water Oxidation
Chemistry of Materials ( IF 7.2 ) Pub Date : 2016-08-09 00:00:00 , DOI: 10.1021/acs.chemmater.6b01522 Yibing Li 1 , Chuan Zhao 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2016-08-09 00:00:00 , DOI: 10.1021/acs.chemmater.6b01522 Yibing Li 1 , Chuan Zhao 1
Affiliation
|
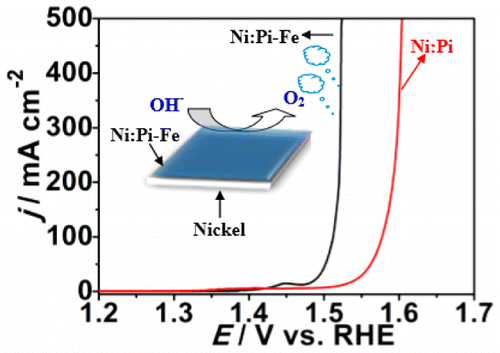
Electrochemical water splitting into hydrogen and oxygen has been regarded as one of the most promising approaches to produce clean hydrogen fuel using electricity generated from renewable energy sources such as solar energy, wind power, or hydropower etc. Recent findings have demonstrated significant potential of nonprecious, nickel-based electrocatalysts as efficient oxygen evolution reaction (OER) to replace traditional ruthenium (Ru) and iridium (Ir)-based precious metal catalysts. Here, for the first time, we report a novel three-dimensional iron-doped nickel phosphate catalyst by stepwise autologous hydrothermal growth of nickel phosphate (Ni:Pi) spontaneously from nickel foam (NF) followed by electrodeposition of iron hydroxide (denoted as Ni:Pi-Fe/NF). Our findings reveal that the incorporated iron could play strong synergistic effects on the OER activities of nickel phosphate in alkaline solution, delivering a current density of 10 mA cm–2 at an extremely small overpotential (η) of 220 mV and extraordinary high current density of 500 mA cm–2 at η = 290 mV in 1 M KOH, which is among the best Ni-based OER electrocatalysts to date. Furthermore, in a concentrated alkaline electrolyte (5 M KOH), the Ni:Pi-Fe/NF electrode can reach a high current density of 1 600 mA cm–2 at an overpotential merely of 332 mV and shows excellent electrocatalytic stability in prolonged bulk water electrolysis, meaning it could highly meet the requirement of the industry alkaline electrolysis system. Mechanism investigations employing X-ray photoelectron spectroscopy (XPS), electrochemical polarization, contact angle measurement, and Raman spectra suggest strong interactions between Ni:Pi and Fe, with nickel oxyhydroxide (NiOOH) being the primary catalytic active site and nickel phosphate facilitating water adsorption. The iron doping changes the local Ni–O environments which synergistically enhance the Ni:Pi-Fe/NF catalytic activity toward OER.
中文翻译:

铁掺杂磷酸镍作为水氧化的协同电催化剂
将电化学水分解成氢和氧已被认为是利用可再生能源(例如太阳能,风能或水力发电等)产生的电能生产清洁氢燃料的最有前途的方法之一。最近的发现表明,非贵金属的巨大潜力,镍基电催化剂作为有效的氧释放反应(OER)替代传统的钌(Ru)和铱(Ir)基贵金属催化剂。在这里,我们首次报道了一种新型的三维掺杂铁的磷酸镍催化剂,该催化剂通过自发地从泡沫镍(NF)中自发逐步进行水热磷酸镍(Ni:Pi)的水热生长,然后电沉积氢氧化铁(表示为Ni) :Pi-Fe / NF)。–2在220 mV的极小过电位(η)和500 mA cm的极高电流密度下–在1 M KOH中在η= 290 mV时在–2时,这是迄今为止最好的Ni基OER电催化剂之一。此外,在浓碱性电解液(5 M KOH)中,Ni:Pi-Fe / NF电极可达到1600 mA cm –2的高电流密度在仅332 mV的超电势下,在长时间的本体水电解中显示出优异的电催化稳定性,这意味着它可以高度满足工业碱性电解系统的要求。使用X射线光电子能谱(XPS),电化学极化,接触角测量和拉曼光谱进行的机理研究表明,Ni:Pi和Fe之间存在强相互作用,其中羟基氧化镍(NiOOH)是主要的催化活性位点,而磷酸镍则促进了水的吸附。铁掺杂改变了局部Ni-O环境,从而协同增强了Ni:Pi-Fe / NF对OER的催化活性。
更新日期:2016-08-09
中文翻译:

铁掺杂磷酸镍作为水氧化的协同电催化剂
将电化学水分解成氢和氧已被认为是利用可再生能源(例如太阳能,风能或水力发电等)产生的电能生产清洁氢燃料的最有前途的方法之一。最近的发现表明,非贵金属的巨大潜力,镍基电催化剂作为有效的氧释放反应(OER)替代传统的钌(Ru)和铱(Ir)基贵金属催化剂。在这里,我们首次报道了一种新型的三维掺杂铁的磷酸镍催化剂,该催化剂通过自发地从泡沫镍(NF)中自发逐步进行水热磷酸镍(Ni:Pi)的水热生长,然后电沉积氢氧化铁(表示为Ni) :Pi-Fe / NF)。–2在220 mV的极小过电位(η)和500 mA cm的极高电流密度下–在1 M KOH中在η= 290 mV时在–2时,这是迄今为止最好的Ni基OER电催化剂之一。此外,在浓碱性电解液(5 M KOH)中,Ni:Pi-Fe / NF电极可达到1600 mA cm –2的高电流密度在仅332 mV的超电势下,在长时间的本体水电解中显示出优异的电催化稳定性,这意味着它可以高度满足工业碱性电解系统的要求。使用X射线光电子能谱(XPS),电化学极化,接触角测量和拉曼光谱进行的机理研究表明,Ni:Pi和Fe之间存在强相互作用,其中羟基氧化镍(NiOOH)是主要的催化活性位点,而磷酸镍则促进了水的吸附。铁掺杂改变了局部Ni-O环境,从而协同增强了Ni:Pi-Fe / NF对OER的催化活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号