当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Helically Arranged Chiral Molecular Nanographenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-07-20 , DOI: 10.1021/jacs.1c05977 Patricia Izquierdo-García 1 , Jesús M Fernández-García 1 , Israel Fernández 1 , Josefina Perles 2 , Nazario Martín 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-07-20 , DOI: 10.1021/jacs.1c05977 Patricia Izquierdo-García 1 , Jesús M Fernández-García 1 , Israel Fernández 1 , Josefina Perles 2 , Nazario Martín 1, 3
Affiliation
|
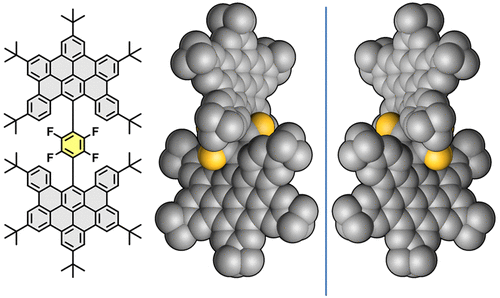
A benchtop solution-phase synthesis of molecular nanographenes composed of two orthogonal dibenzo[fg,ij]phenanthro[9,10,1,2,3-pqrst]pentaphene (DBPP) moieties covalently connected through a tetrafluorobenzene ring is described. The helical arrangement of these three covalently linked molecular fragments leads to the existence of a chiral axis which gives rise to a racemic mixture, even with the molecular moieties being symmetrically substituted. X-ray diffraction studies show that both enantiomers cocrystallize in a single crystal, and the racemic mixture can be resolved by chiral HPLC. Asymmetric substitution in DBPP moieties affords a pair of diastereoisomers whose rotational isomerization has been studied by 1H NMR. Additionally, the electrochemical and photophysical properties derived from these new molecular nanographenes reveal an electroactive character and a significant fluorescent behavior.
中文翻译:

螺旋排列的手性分子纳米石墨烯
描述了由通过四氟苯环共价连接的两个正交二苯并[fg,ij]菲[9,10,1,2,3-pqrst]五苯 (DBPP) 部分组成的分子纳米石墨烯的台式溶液相合成。这三个共价连接的分子片段的螺旋排列导致了手性轴的存在,从而产生了外消旋混合物,即使分子部分被对称取代。X 射线衍射研究表明,两种对映体均以单晶形式共结晶,外消旋混合物可通过手性 HPLC 分离。DBPP 部分中的不对称取代提供了一对非对映异构体,其旋转异构化已由1核磁共振氢谱。此外,源自这些新分子纳米石墨烯的电化学和光物理性质揭示了电活性特性和显着的荧光行为。
更新日期:2021-08-04
中文翻译:

螺旋排列的手性分子纳米石墨烯
描述了由通过四氟苯环共价连接的两个正交二苯并[fg,ij]菲[9,10,1,2,3-pqrst]五苯 (DBPP) 部分组成的分子纳米石墨烯的台式溶液相合成。这三个共价连接的分子片段的螺旋排列导致了手性轴的存在,从而产生了外消旋混合物,即使分子部分被对称取代。X 射线衍射研究表明,两种对映体均以单晶形式共结晶,外消旋混合物可通过手性 HPLC 分离。DBPP 部分中的不对称取代提供了一对非对映异构体,其旋转异构化已由1核磁共振氢谱。此外,源自这些新分子纳米石墨烯的电化学和光物理性质揭示了电活性特性和显着的荧光行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号