Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-07-20 , DOI: 10.1016/j.molstruc.2021.131138 Ahmed H. Abdelazeem 1, 2 , Alaa M. Alqahtani 3 , Hany H. Arab 4 , Ahmed M. Gouda 1 , Asmaa G. Safi El-Din 1
|
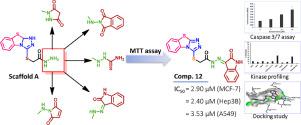
In the current study, we report the synthesis and cytotoxic evaluation of a new series of S-benzo[4,5]thiazolo[2,3-c][1,2,4]triazole-based derivatives 8-12. Cytotoxicity of the new compounds was investigated in A549, MCF-7, and Hep3B cancer cell lines. Among these derivatives, compound 12 bearing an isatin moiety was the most active derivative (IC50 = 2.40-3.53 µM). A mechanistic study of compound 12 was performed using the kinase profiling test to explore its inhibitory activity against 10 types of the oncogenic kinases and the potential activation of caspase 3/7 enzymes. The results revealed that compound 12 showed moderate inhibition of the EGFR and LCK kinases. Moreover, compound 12 also activated caspase-3/7 in A549 cells. The docking study of compound 12 into EGFR ATP-active site revealed that it fits nicely with good binding affinity. Together, the results indicated that compound 12 could serve as a good lead for developing new potential anticancer agents.
中文翻译:

新型苯并[4,5]噻唑并[2,3-C][1,2,4]三唑:设计、合成、抗癌评估、激酶分析和分子对接研究
在目前的研究中,我们报告了一系列新的S-苯并 [4,5] 噻唑并 [2,3- c ][1,2,4] 三唑衍生物8-12的合成和细胞毒性评估。在 A549、MCF-7 和 Hep3B 癌细胞系中研究了新化合物的细胞毒性。在这些衍生物中,带有靛红部分的化合物12是最活跃的衍生物 (IC 50 = 2.40-3.53 µM)。使用激酶分析测试对化合物12进行了机理研究,以探索其对 10 种致癌激酶的抑制活性和半胱天冬酶 3/7 酶的潜在激活。结果表明,化合物12显示对 EGFR 和 LCK 激酶的中度抑制。此外,化合物12还激活了 A549 细胞中的 caspase-3/7。化合物12与 EGFR ATP 活性位点的对接研究表明,它非常适合具有良好的结合亲和力。总之,结果表明化合物12可以作为开发新的潜在抗癌剂的良好先导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号