当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Unified Approach for the Enantioselective Synthesis of the Brominated Chamigrene Sesquiterpenes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2016-08-10 , DOI: 10.1002/anie.201605722 Alexander J Burckle 1 , Vasil H Vasilev 1 , Noah Z Burns 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2016-08-10 , DOI: 10.1002/anie.201605722 Alexander J Burckle 1 , Vasil H Vasilev 1 , Noah Z Burns 1
Affiliation
|
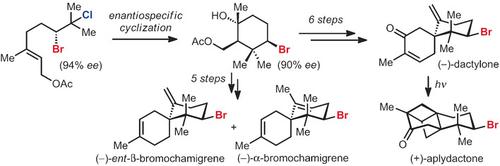
The brominated chamigrene sesquiterpenes constitute a large subclass of bromocyclohexane‐containing natural products, yet no general enantioselective strategy for the synthesis of these small molecules exists. Herein we report a general strategy for accessing this family of secondary metabolites, including the enantioselective synthesis of (−)‐α‐ and (−)‐ent‐β‐bromochamigrene, (−)‐dactylone, and (+)‐aplydactone. Access to these molecules is enabled by a stereospecific bromopolyene cyclization initiated by the solvolysis of an enantiomerically enriched vicinal bromochloride.
中文翻译:

溴化氨基倍半萜对映选择性合成的统一方法
溴化的半萜倍半萜构成了含溴环己烷的天然产物的一大类,但不存在合成这些小分子的通用对映选择性策略。在此,我们报告了获取该次级代谢物家族的一般策略,包括(−)-α-和(−)- ent -β-bromochamigrene、(−)-dactylone和(+)-aplydactone的对映选择性合成。通过对映体富集的邻位溴氯化物的溶剂分解引发立体特异性溴多烯环化,从而能够获得这些分子。
更新日期:2016-08-10
中文翻译:

溴化氨基倍半萜对映选择性合成的统一方法
溴化的半萜倍半萜构成了含溴环己烷的天然产物的一大类,但不存在合成这些小分子的通用对映选择性策略。在此,我们报告了获取该次级代谢物家族的一般策略,包括(−)-α-和(−)- ent -β-bromochamigrene、(−)-dactylone和(+)-aplydactone的对映选择性合成。通过对映体富集的邻位溴氯化物的溶剂分解引发立体特异性溴多烯环化,从而能够获得这些分子。































 京公网安备 11010802027423号
京公网安备 11010802027423号