当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrocyclization by Rhodium-Catalyzed Cross-Cyclotrimerization of L-Shaped Diynes with Di-tert-butyl Acetylenedicarboxylate: Effect of Bent Linkers of Diynes
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-08-23 , DOI: 10.1002/ejoc.201600909 Shuhei Nishigaki 1 , Yuta Miyauchi 1 , Keiichi Noguchi 2 , Hideto Ito 3 , Kenichiro Itami 3 , Yu Shibata 1 , Ken Tanaka 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-08-23 , DOI: 10.1002/ejoc.201600909 Shuhei Nishigaki 1 , Yuta Miyauchi 1 , Keiichi Noguchi 2 , Hideto Ito 3 , Kenichiro Itami 3 , Yu Shibata 1 , Ken Tanaka 1
Affiliation
|
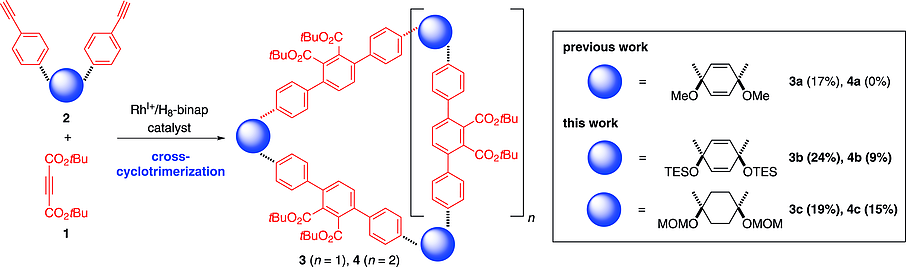
The effects of bent linkers in diynes on the efficiency and selectivity of intermolecular macrocyclization by the rhodium-catalyzed sequential cross-cyclotrimerization of L-shaped diynes and di-tert-butyl acetylenedicarboxylate was investigated. A bulky triethylsilyl-protected cyclohexa-1,4-dienediol-linked diyne reacted with di-tert-butyl acetylenedicarboxylate to give the corresponding 16-membered macrocycle as well as the 12-membered macrocycle. Aromatization of the thus-obtained 12-membered macrocycle through desilylation followed by reductive aromatization with SnCl2 and HCl afforded a [12]cycloparaphenylenehexacarboxylate. The use of a conformationally flexible methoxymethyl-protected cyclohexanediol-linked diyne increased the yield of the corresponding 16-membered macrocycle, although oxidative aromatization leading to the corresponding cycloparaphenylenes was unsuccessful.
中文翻译:

L-形二炔与乙炔二甲酸二叔丁酯的铑催化交叉环三聚反应的大环化:二炔弯曲接头的影响
研究了二炔中的弯曲连接基对通过铑催化的 L 型二炔和乙炔二羧酸二叔丁基酯的顺序交叉环三聚反应进行的分子间大环化的效率和选择性的影响。庞大的三乙基甲硅烷基保护的环六-1,4-二烯二醇连接的二炔与乙炔二羧酸二叔丁酯反应生成相应的 16 元大环和 12 元大环。由此获得的 12 元大环通过脱甲硅烷基化进行芳构化,然后用 SnCl2 和 HCl 进行还原芳构化,得到 [12] 环对亚苯基六羧酸酯。使用构象灵活的甲氧基甲基保护的环己二醇连接的二炔增加了相应的 16 元大环的产率,
更新日期:2016-08-23
中文翻译:

L-形二炔与乙炔二甲酸二叔丁酯的铑催化交叉环三聚反应的大环化:二炔弯曲接头的影响
研究了二炔中的弯曲连接基对通过铑催化的 L 型二炔和乙炔二羧酸二叔丁基酯的顺序交叉环三聚反应进行的分子间大环化的效率和选择性的影响。庞大的三乙基甲硅烷基保护的环六-1,4-二烯二醇连接的二炔与乙炔二羧酸二叔丁酯反应生成相应的 16 元大环和 12 元大环。由此获得的 12 元大环通过脱甲硅烷基化进行芳构化,然后用 SnCl2 和 HCl 进行还原芳构化,得到 [12] 环对亚苯基六羧酸酯。使用构象灵活的甲氧基甲基保护的环己二醇连接的二炔增加了相应的 16 元大环的产率,















































 京公网安备 11010802027423号
京公网安备 11010802027423号