Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-07-16 , DOI: 10.1016/j.bioorg.2021.105176 Mohamed H Hekal 1 , Paula S Farag 1 , Magdy M Hemdan 1 , Wael M El-Sayed 2
|
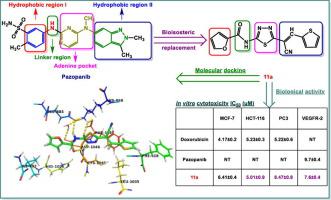
The present study reports the synthesis and biological evaluation of a new series of novel N-(1,3,4-thiadiazol-2-yl)furan-2-carboxamide derivatives. The reactions were executed under both conventional and microwave irradiation conditions. An enhancement in the synthetic yields and rates was observed when the reactions were carried out under the microwave compared with the classical conditions. The structures of the products were ascertained by different analytical and spectral analyses. The antiproliferative activities were evaluated against three human epithelial cell lines; breast (MCF-7), colon (HCT-116), and prostate (PC-3) using MTT assay technique and doxorubicin was utilized as a reference drug. Besides, molecular docking studies were also performed and the vascular endothelial growth factor recptor-2 (VEGFR-2) was identified as a potential molecular target. Compounds 6, 7, 11a, 11b, 12, 14, and 16 showed promising antiproliferative activity against the three cancer cell lines investigated. Compounds 2 and 15b had significant antiproliferative activities against only colon and breast cells but not against the prostate cells. All the active antiproliferative compounds were highly selective. All the active antiproliferative compounds were good inhibitors of the VEGFR-2 at 7.4–11.5 nM compared with Pazopanib. Compound 7 with the most favorable orientation to the VEGFR-2 from the docking studies, was also the best inhibitor of the receptor. The antiproliferative activity of these compounds is in partial caused by their ability to inhibit the VEGFR-2 and since other molecular targets were not examined, other possibilities cannot be ruled out.
中文翻译:

新的 N-(1,3,4-thiadiazol-2-yl)furan-2-carboxamide 衍生物作为 VEGFR-2 的潜在抑制剂
本研究报告了一系列新的新型N的合成和生物学评价-(1,3,4-噻二唑-2-基)呋喃-2-甲酰胺衍生物。反应在常规和微波辐射条件下进行。与经典条件相比,当反应在微波下进行时,观察到合成产率和速率的提高。通过不同的分析和光谱分析确定了产物的结构。针对三种人类上皮细胞系评估了抗增殖活性;乳腺 (MCF-7)、结肠 (HCT-116) 和前列腺 (PC-3) 使用 MTT 测定技术,多柔比星用作参考药物。此外,还进行了分子对接研究,血管内皮生长因子受体-2 (VEGFR-2) 被确定为潜在的分子靶点。化合物6 , 7、11a、11b、12、14和16对所研究的三种癌细胞系显示出有希望的抗增殖活性。化合物2和15b仅对结肠细胞和乳腺细胞具有显着的抗增殖活性,但对前列腺细胞没有作用。所有活性抗增殖化合物都是高度选择性的。与帕唑帕尼相比,所有活性抗增殖化合物都是 7.4-11.5 nM 的 VEGFR-2 的良好抑制剂。化合物7对接研究中对 VEGFR-2 最有利的方向,也是该受体的最佳抑制剂。这些化合物的抗增殖活性部分是由它们抑制 VEGFR-2 的能力引起的,并且由于未检查其他分子靶标,因此不能排除其他可能性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号