当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyhalogenated Bicyclo[1.1.1]pentane-1,3-dicarboxylic Acids
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-07-16 , DOI: 10.1021/acs.joc.1c01020 Thi Phuong Le 1 , Igor Rončević 1 , Martin Dračínský 1 , Ivana Císařová 2 , Veronika Šolínová 1 , Václav Kašička 1 , Jiří Kaleta 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-07-16 , DOI: 10.1021/acs.joc.1c01020 Thi Phuong Le 1 , Igor Rončević 1 , Martin Dračínský 1 , Ivana Císařová 2 , Veronika Šolínová 1 , Václav Kašička 1 , Jiří Kaleta 1
Affiliation
|
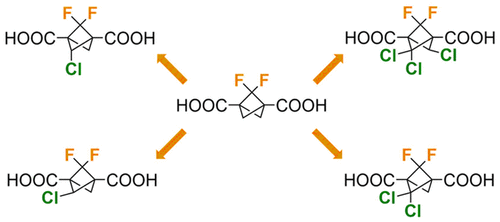
Herein we report the highly selective radical chlorination of 2,2-difluorobicyclo[1.1.1]pentane-1,3-dicarboxylic acid. Together with radical hydrodechlorination by TMS3SiH, four new bicyclo[1.1.1]pentane cages carrying two fluorine and one to three chlorine atoms in bridge positions have been obtained. The exact positions of all halogen atoms have been confirmed by X-ray diffraction. The acidity constants (pKa) for all new derivatives have been determined by capillary electrophoresis, and these experimental values show excellent agreement with pKas predicted by DFT methods. Extensive DFT calculations have been used to rationalize the selective formation of four out of nine possible F2Cl1–4 isomers of bridge-halogenated bicyclo[1.1.1]pentanes and to obtain relative strain energies for all possible isomers.
中文翻译:

多卤化双环[1.1.1]戊烷-1,3-二羧酸
在此,我们报告了 2,2-二氟双环 [1.1.1] 戊烷-1,3-二羧酸的高选择性自由基氯化。与 TMS 3 SiH 的自由基加氢脱氯一起,获得了四个新的双环 [1.1.1] 戊烷笼,在桥位上携带两个氟和一到三个氯原子。所有卤素原子的确切位置已通过 X 射线衍射确认。所有新衍生物的酸度常数 (p K a ) 都通过毛细管电泳确定,这些实验值与DFT 方法预测的p K a s非常吻合。广泛的 DFT 计算已被用于合理化九种可能的 F 2 Cl 1-4中四种的选择性形成 桥卤化双环[1.1.1]戊烷的异构体,并获得所有可能异构体的相对应变能。
更新日期:2021-08-07
中文翻译:

多卤化双环[1.1.1]戊烷-1,3-二羧酸
在此,我们报告了 2,2-二氟双环 [1.1.1] 戊烷-1,3-二羧酸的高选择性自由基氯化。与 TMS 3 SiH 的自由基加氢脱氯一起,获得了四个新的双环 [1.1.1] 戊烷笼,在桥位上携带两个氟和一到三个氯原子。所有卤素原子的确切位置已通过 X 射线衍射确认。所有新衍生物的酸度常数 (p K a ) 都通过毛细管电泳确定,这些实验值与DFT 方法预测的p K a s非常吻合。广泛的 DFT 计算已被用于合理化九种可能的 F 2 Cl 1-4中四种的选择性形成 桥卤化双环[1.1.1]戊烷的异构体,并获得所有可能异构体的相对应变能。































 京公网安备 11010802027423号
京公网安备 11010802027423号