Tetrahedron ( IF 2.1 ) Pub Date : 2021-07-15 , DOI: 10.1016/j.tet.2021.132329 Shunya Morita 1 , Tomoyuki Yoshimura 1 , Jun-ichi Matsuo 1
|
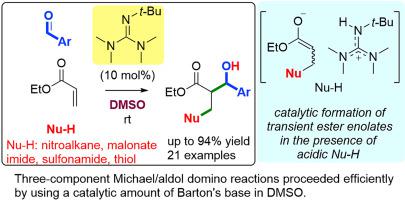
In DMSO, a catalytic amount of Barton's base (2-t-butyl-1,1,3,3-tetramethylguanidine, BTMG) effectively catalyzed intermolecular three-component reactions of α,β-unsaturated esters, aldehydes, and carbon-, sulfur-, or nitrogen-pronucleophiles to give three-component addition products with the formation of two new σ-bonds: pronucleophiles and aldehydes reacted with α,β-unsaturated esters at their β-positions and α-positions, respectively. Mechanism studies suggested that these reactions proceeded by the first intermolecular Michael addition of anionic nucleophiles that were formed from pronucleophiles with a catalytic amount of BTMG, followed by intermolecular aldol reactions of transient ester enolates even in the presence of more than stoichiometric amounts of acidic pronucleophiles. High nucleophilicity over Brønsted basicity of transient enolates in polar solvents was observed for transient ester enolates rather than ketone enolates.
中文翻译:

α,β-不饱和酯、芳香醛和各种亲核试剂在 DMSO 中催化量的胍碱促进的分子间多米诺迈克尔/羟醛反应
在 DMSO 中,催化量的巴顿碱 (2- t-丁基-1,1,3,3-四甲基胍,BTMG)有效催化α,β-不饱和酯、醛和碳-、硫-或氮-亲核试剂的分子间三组分反应,得到三组分加成产物形成两个新的 σ 键:亲核试剂和醛分别在其 β 位和 α 位与 α,β-不饱和酯反应。机理研究表明,这些反应是通过第一次分子间迈克尔加成的阴离子亲核试剂与催化量的 BTMG 形成的阴离子亲核试剂进行的,然后是瞬时酯烯醇化物的分子间醛醇反应,即使在超过化学计量的酸性亲核试剂存在下。































 京公网安备 11010802027423号
京公网安备 11010802027423号