International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2021-07-15 , DOI: 10.1016/j.ijhydene.2021.06.152 Jinrui Guo 1 , Jing Tian 1 , Jinhua Deng 1 , Xinyu Yang 1 , Binghui Duan 1 , Yong Liu 1, 2
|
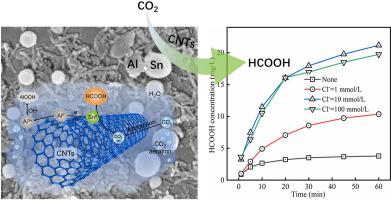
Electrochemical reduction and photocatalytic reduction of CO2 have attracted more and more attention, but they also face the problem of low utilization efficiency of electricity and solar energy. In this study, a new strategy of applying a novel Al–Sn-CNTs composite in electrochemical corrosion process was proposed to reduce CO2 without additional electricity and light. In the Al–Sn-CNTs/CO2 system, micro galvanic cell with Al as anode and Sn or CNTs as cathode was formed, and CO2 was reduced to formic acid on the cathode surface. The cumulative formic acid concentration of the Al–Sn-CNTs/CO2 system achieved 21.18 mg/L within 60 min under the conditions of initial pH 9.0, Cl− concentration 10 mmol/L, and Al–Sn-CNTs composite dosage 2 g/L. Based on the morphology, crystal structure and electrochemical test results of the Al–Sn-CNTs composite, a possible mechanism of CO2 reduction to formic acid in the Al–Sn-CNTs/CO2 system was proposed.
中文翻译:

Al-Sn-CNTs复合材料催化还原CO2合成甲酸
CO 2 的电化学还原和光催化还原越来越受到关注,但也面临着电能和太阳能利用效率低的问题。在这项研究中,提出了一种在电化学腐蚀过程中应用新型 Al-Sn-CNTs 复合材料的新策略,以减少 CO 2而不需要额外的电力和光。在Al-Sn-CNTs/CO 2体系中,形成了以Al为阳极,以Sn或CNTs为阴极的微型原电池,CO 2在阴极表面被还原为甲酸。Al-Sn-CNTs/CO 2体系的累积甲酸浓度在初始 pH 9.0、Cl -条件下在 60 分钟内达到 21.18 mg/L浓度为 10 mmol/L,Al-Sn-CNTs 复合剂量为 2 g/L。基于Al-Sn-CNTs复合材料的形貌、晶体结构和电化学测试结果,提出了Al-Sn-CNTs/CO 2体系中CO 2还原为甲酸的可能机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号