Chem Catalysis ( IF 11.5 ) Pub Date : 2021-06-14 , DOI: 10.1016/j.checat.2021.05.007 Guoying Zhang , Torsten Irrgang , Martin Schlagbauer , Rhett Kempe
|
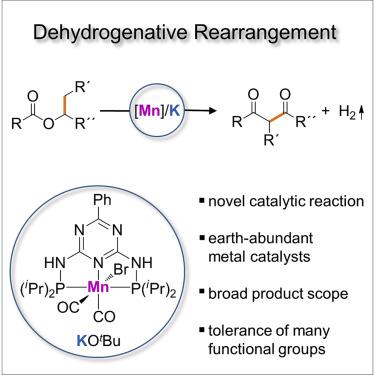
Catalytic reactions that convert green or sustainable starting materials into important classes of chemical compounds, generate hydrogen, and are mediated by Earth-abundant element catalysts are highly desirable. Here, we report on a catalytic dehydrogenative rearrangement of esters into 1,3-diketones. Esters are green or sustainable and inexpensive starting materials that are available in great diversity, and 1,3-diketones are highly attractive building blocks for organic synthesis. The concerted interaction of a base-acid catalyst and a dehydrogenation catalyst mediates our reaction. The dehydrogenation catalyst is based on manganese, and the hydrogen formed can be liberated and is the only by-product formed.
中文翻译:

通过释放氢从酯合成 1,3-二酮
非常需要将绿色或可持续原材料转化为重要类别的化合物、产生氢气并由地球上丰富的元素催化剂介导的催化反应。在这里,我们报告了酯催化脱氢重排成 1,3-二酮。酯类是绿色或可持续且价格低廉的起始材料,种类繁多,而 1,3-二酮是非常有吸引力的有机合成基础材料。碱-酸催化剂和脱氢催化剂的协同相互作用介导了我们的反应。脱氢催化剂以锰为基础,形成的氢气可以释放出来,是唯一形成的副产物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号