Chem Catalysis ( IF 11.5 ) Pub Date : 2021-05-10 , DOI: 10.1016/j.checat.2021.04.001 Siyang Nie , Liang Wu , Lingci Zhao , Xiao Zheng , Shize Yang , Pengfei Zhang
|
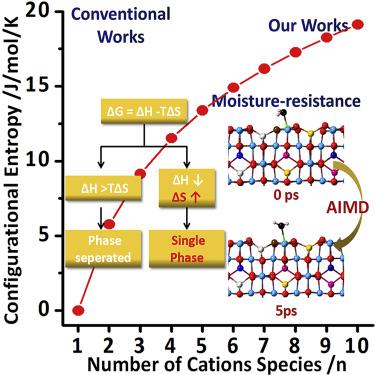
High-entropy ceramics (HECs) are novel materials with unexpected chemistry. However, HEC-based catalysis or chemistry has rarely been studied before. Herein, porous high-entropy MgAl2O4 (HE-MgAl2O4) (up to denary metals: Mg, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn) was prepared (SBET = 139 m2/g). The synthetic chemistry, the tolerance law of different metals, and the thermodynamic rule for crystallization have been well studied. The comparison of binary-, ternary-, quaternary-, quinary-, and denary-MgAl2O4 highlighted the key role of entropy, which forces cations with intrinsic different radii to occupy similar sites of spinel. Interestingly, high entropy naturally endows the HE-MgAl2O4 catalyst with exceptional stability even when operated at high-temperature moisture (350°C) for 24 h. Then, ab initio molecular dynamics studies proved that the thermal stability of HE-MgAl2O4 originated from the entropy stabilization effect and kinetically stabilized structure. Moreover, CH4 oxidation with 10 vol % steam in feeding gas was performed, and the pristine activity was preserved by the HE-MgAl2O4 catalyst over continuous operation (600°C, 100 h).
中文翻译:

熵驱动化学揭示了高度稳定的二元 MgAl2O4 型催化剂
高熵陶瓷 (HEC) 是一种具有意想不到的化学性质的新型材料。然而,之前很少研究基于 HEC 的催化或化学。在此,制备了多孔高熵 MgAl 2 O 4 (HE-MgAl 2 O 4 )(最多十元金属:Mg、Ti、V、Cr、Mn、Fe、Co、Ni、Cu 和 Zn)(S BET = 139 m 2 /g)。对合成化学、不同金属的耐受规律以及结晶的热力学规律进行了很好的研究。二元、三元、四元、五元和二元-MgAl 2 O 4 的比较强调了熵的关键作用,它迫使具有内在不同半径的阳离子占据尖晶石的相似位置。有趣的是,即使在高温湿气 (350°C) 下运行 24 小时,高熵自然赋予 HE-MgAl 2 O 4催化剂卓越的稳定性。然后,从头分子动力学研究证明,HE-MgAl 2 O 4的热稳定性源于熵稳定效应和动力学稳定结构。此外,在原料气中用 10 vol% 的蒸汽进行CH 4氧化,HE-MgAl 2 O 4保持了原始活性 催化剂持续运行(600°C,100 小时)。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号