当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidation of Roxarsone Coupled with Sorptive Removal of the Inorganic Arsenic Released by Iron–Carbon (Fe–C) Microelectrolysis
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-07-13 , DOI: 10.1021/acsestengg.1c00129 Xiande Xie 1, 2 , Hefa Cheng 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-07-13 , DOI: 10.1021/acsestengg.1c00129 Xiande Xie 1, 2 , Hefa Cheng 1
Affiliation
|
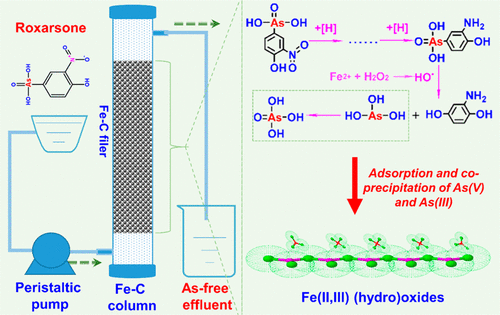
Iron–carbon (Fe–C) microelectrolysis was proposed as a novel method for simultaneous degradation of a model phenylarsenic compound, roxarsone (ROX), in manure leachate and removal of the inorganic arsenic released. The initial rate equation for ROX degradation was determined as rinit = 1.55[ROX]1.0[Fe–C]0.9[H+]0.5 (μM/min), at the initial solution pH of 2.0–5.0 and in the presence of overdosed Fe–C filler. A unique advantage of Fe–C microelectrolysis process is that ROX was first reduced to 3-amino-4-hydroxyphenylarsenic acid by the atomic hydrogen generated by cathodic reduction under acidic conditions, which facilitated subsequent oxidative attack by HO•. The iron (hydro)oxide precipitate formed from iron corrosion effectively captured the inorganic arsenic released, reducing the residual arsenic level in the treated water and swine manure leachate to <100 μg/L (standard for irrigation water in China), while the treatment performance was barely affected by the major constitutes of water matrix. The residual level of arsenic in the effluent of a Fe–C microelectrolysis column could be kept consistently below 100 μg/L for 5 months with proper replenishment of the Fe–C filler. The concurrent occurrence of reduction and oxidation reactions and the in situ generation of iron (hydro)oxides make Fe–C microelectrolysis process particularly efficient for treating nitroaromatic compounds and organoarsenicals.
更新日期:2021-09-10






































 京公网安备 11010802027423号
京公网安备 11010802027423号