当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible‐Light Photoredox‐Catalyzed Semipinacol‐Type Rearrangement: Trifluoromethylation/Ring Expansion by a Radical–Polar Mechanism
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2015-06-01 , DOI: 10.1002/anie.201503210 Basudev Sahoo , Jun-Long Li , Frank Glorius
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2015-06-01 , DOI: 10.1002/anie.201503210 Basudev Sahoo , Jun-Long Li , Frank Glorius
|
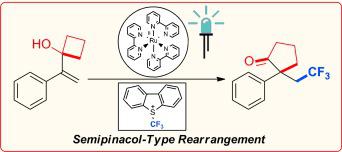
A visible‐light‐mediated photoredox‐catalyzed semipinacol‐type rearrangement proceeding via 1,2 alkyl migration was developed. In this transformation, trifluoromethylation of the CC bond of α‐(1‐hydroxycycloalkyl)‐substituted styrene derivatives is followed by ring expansion of the 1‐hydroxycycloalkyl group to deliver novel cycloalkanones with all‐carbon quaternary centers. The reaction proceeds via a radical–polar mechanism, with trifluoromethylation (radical) and ring expansion (ionic) occurring in the same transformation.
中文翻译:

可见光光氧化还原催化的半松果酚型重排:通过自由基-极性机理的三氟甲基化/环扩展
通过1,2烷基转移的可见光介导的光氧化还原催化的半频哪醇型重排得以发展。在此转化过程中,α-(1-羟基环烷基)取代的苯乙烯衍生物的CC键进行三氟甲基化,然后1-羟基环烷基进行环扩环,以提供具有全碳季中心的新型环烷酮。该反应通过自由基-极性机理进行,三氟甲基化(自由基)和环膨胀(离子)以相同的转化发生。
更新日期:2015-06-01
中文翻译:

可见光光氧化还原催化的半松果酚型重排:通过自由基-极性机理的三氟甲基化/环扩展
通过1,2烷基转移的可见光介导的光氧化还原催化的半频哪醇型重排得以发展。在此转化过程中,α-(1-羟基环烷基)取代的苯乙烯衍生物的CC键进行三氟甲基化,然后1-羟基环烷基进行环扩环,以提供具有全碳季中心的新型环烷酮。该反应通过自由基-极性机理进行,三氟甲基化(自由基)和环膨胀(离子)以相同的转化发生。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号