当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immune/Hypoxic Tumor Microenvironment Regulation-Enhanced Photodynamic Treatment Realized by pH-Responsive Phase Transition-Targeting Nanobubbles
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-07-08 , DOI: 10.1021/acsami.1c07323 Meng Zhao 1 , Xupeng Yang 1 , Hao Fu 1 , Chuanrong Chen 1 , Yanhua Zhang 1 , Zhihua Wu 1 , Yourong Duan 1 , Ying Sun 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-07-08 , DOI: 10.1021/acsami.1c07323 Meng Zhao 1 , Xupeng Yang 1 , Hao Fu 1 , Chuanrong Chen 1 , Yanhua Zhang 1 , Zhihua Wu 1 , Yourong Duan 1 , Ying Sun 1
Affiliation
|
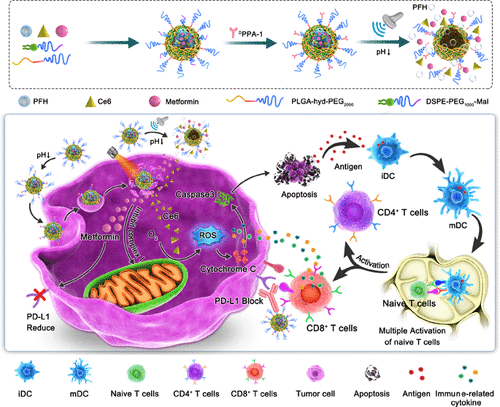
Due to a special pathological type of triple-negative breast cancer (TNBC) and the lack of expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her 2), targeted therapies are not effective. The lack of effective treatment drugs and insensitivity to the current single-treatment methods are the biggest problems that we face with the TNBC treatment. Therefore, new strategies to achieve selective treatment and further visual efficacy evaluation will be powerful tools against TNBC. Herein, a novel tumor-targeted nanosized ultrasound contrast nanobubble loaded with chlorin e6 (Ce6), metformin (MET), and perfluorohexane (PFH) and covalently connected to the anti-PD-L1 peptide (DPPA-1) in the outer shell was fabricated. When accumulated in acidic tumor tissues, poly(ethylene glycol) (PEG) ligands detach, and DPPA-1 is exposed for programmed death-ligand 1 (PD-L1) targeting and blocking. The released metformin can relieve hypoxia by inhibiting mitochondrial complex I in the tumor microenvironment (TME) and enhance the therapeutic efficacy of Ce6 while synergizing with DPPA-1 by reducing PD-L1 expression. More significantly, photodynamic therapy (PDT) using multifunctional tumor-targeted nanosized ultrasound contrast agents (PD-L1-targeted pH-sensitive chlorin e6 (Ce6) and metformin (MET) drug-loaded phase transitional nanoparticles (Ce6/MET NPs-DPPA-1)) combined with PD-L1 checkpoint blocking can not only reduce tumor-mediated immunosuppression but also produce strong antitumor immunity. This finding provides a new idea for constructing multifunctional TNBC therapeutic nanoagents.
中文翻译:

通过 pH 响应相变靶向纳米气泡实现的免疫/缺氧肿瘤微环境调节增强光动力治疗
由于三阴性乳腺癌 (TNBC) 的特殊病理类型以及雌激素受体 (ER)、孕激素受体 (PR) 和人表皮生长因子受体 2 (Her 2) 的表达缺失,靶向治疗不有效的。缺乏有效的治疗药物和对目前单一治疗方法的不敏感是我们在 TNBC 治疗中面临的最大问题。因此,实现选择性治疗和进一步视觉效果评估的新策略将成为对抗 TNBC 的有力工具。在此,一种新型的肿瘤靶向纳米超声造影剂纳米气泡装载有二氢卟酚 e6 (Ce6)、二甲双胍 (MET) 和全氟己烷 (PFH),并与抗 PD-L1 肽 ( D外壳中的 PPA-1)。当在酸性肿瘤组织中积累时,聚乙二醇 (PEG) 配体分离,D PPA-1 暴露于程序性死亡配体 1 (PD-L1) 靶向和阻断。释放的二甲双胍可通过抑制肿瘤微环境(TME)中的线粒体复合物 I 缓解缺氧,增强 Ce6 的治疗效果,同时通过降低 PD-L1 表达与D PPA-1协同作用。更重要的是,使用多功能肿瘤靶向纳米超声造影剂(PD-L1靶向pH敏感二氢卟酚e6(Ce6)和二甲双胍(MET)载药相变纳米颗粒(Ce6/MET NPs- D)的光动力疗法(PDT)PPA-1))结合PD-L1检查点阻断,不仅可以减轻肿瘤介导的免疫抑制,还可以产生强大的抗肿瘤免疫。这一发现为构建多功能 TNBC 治疗纳米药物提供了新思路。
更新日期:2021-07-21
中文翻译:

通过 pH 响应相变靶向纳米气泡实现的免疫/缺氧肿瘤微环境调节增强光动力治疗
由于三阴性乳腺癌 (TNBC) 的特殊病理类型以及雌激素受体 (ER)、孕激素受体 (PR) 和人表皮生长因子受体 2 (Her 2) 的表达缺失,靶向治疗不有效的。缺乏有效的治疗药物和对目前单一治疗方法的不敏感是我们在 TNBC 治疗中面临的最大问题。因此,实现选择性治疗和进一步视觉效果评估的新策略将成为对抗 TNBC 的有力工具。在此,一种新型的肿瘤靶向纳米超声造影剂纳米气泡装载有二氢卟酚 e6 (Ce6)、二甲双胍 (MET) 和全氟己烷 (PFH),并与抗 PD-L1 肽 ( D外壳中的 PPA-1)。当在酸性肿瘤组织中积累时,聚乙二醇 (PEG) 配体分离,D PPA-1 暴露于程序性死亡配体 1 (PD-L1) 靶向和阻断。释放的二甲双胍可通过抑制肿瘤微环境(TME)中的线粒体复合物 I 缓解缺氧,增强 Ce6 的治疗效果,同时通过降低 PD-L1 表达与D PPA-1协同作用。更重要的是,使用多功能肿瘤靶向纳米超声造影剂(PD-L1靶向pH敏感二氢卟酚e6(Ce6)和二甲双胍(MET)载药相变纳米颗粒(Ce6/MET NPs- D)的光动力疗法(PDT)PPA-1))结合PD-L1检查点阻断,不仅可以减轻肿瘤介导的免疫抑制,还可以产生强大的抗肿瘤免疫。这一发现为构建多功能 TNBC 治疗纳米药物提供了新思路。































 京公网安备 11010802027423号
京公网安备 11010802027423号