当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine-Mediated Synthesis of 2-(Methylthio)-4H-chromen-4-ones and Study of Their Halogenation Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-07-07 , DOI: 10.1021/acs.joc.1c00788 Amr Elagamy 1 , Ranjay Shaw 1 , Chandan Shah 1 , Ramendra Pratap 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-07-07 , DOI: 10.1021/acs.joc.1c00788 Amr Elagamy 1 , Ranjay Shaw 1 , Chandan Shah 1 , Ramendra Pratap 1
Affiliation
|
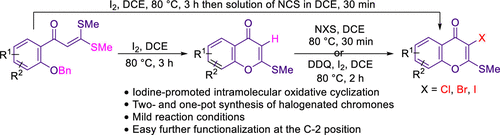
An efficient iodine-mediated method is developed for the synthesis of functionalized 2-(methylthio)-4H-chromen-4-ones by intramolecular cyclization of easily accessible 1-(2-benzyloxy-aryl)-3,3-bis-methylsulfanyl-propenones. The synthesized chromen-4-ones turn out to be a key precursor for various kinds of chemical reactions. Mechanistically, we observed that iodine-mediated intramolecular cyclization of ketene dithioacetal proceeded through a radical pathway. 3-Halo-2-(methylthio)-4H-chromen-4-ones were achieved via various two- or one-pot halogenation approaches.
中文翻译:

碘介导的 2-(甲硫基)-4 H -chromen-4-ones 的合成及其卤化反应研究
开发了一种有效的碘介导方法,用于通过容易获得的 1-(2-苄氧基-芳基)-3,3-双-甲基硫烷基的分子内环化合成功能化的 2-(甲硫基)-4 H-色烯-4-酮-丙烯酮。合成的 chromen-4-ones 是各种化学反应的关键前体。从机制上讲,我们观察到碘介导的二硫代乙缩醛分子内环化是通过自由基途径进行的。3-Halo-2-(methylthio)-4 H -chromen-4-ones 是通过各种两锅或一锅卤化方法实现的。
更新日期:2021-07-16
中文翻译:

碘介导的 2-(甲硫基)-4 H -chromen-4-ones 的合成及其卤化反应研究
开发了一种有效的碘介导方法,用于通过容易获得的 1-(2-苄氧基-芳基)-3,3-双-甲基硫烷基的分子内环化合成功能化的 2-(甲硫基)-4 H-色烯-4-酮-丙烯酮。合成的 chromen-4-ones 是各种化学反应的关键前体。从机制上讲,我们观察到碘介导的二硫代乙缩醛分子内环化是通过自由基途径进行的。3-Halo-2-(methylthio)-4 H -chromen-4-ones 是通过各种两锅或一锅卤化方法实现的。































 京公网安备 11010802027423号
京公网安备 11010802027423号