Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2021-07-08 , DOI: 10.1016/j.jcmgh.2021.06.026
Maria Rivas 1 , Michael E Johnston 2 , Ruhi Gulati 3 , Meenasri Kumbaji 3 , Talita Ferreira Margues Aguiar 4 , Lubov Timchenko 5 , Ana Krepischi 4 , Soona Shin 2 , Alexander Bondoc 3 , Gregory Tiao 2 , James Geller 6 , Nikolai Timchenko 2
|
Background & Aims
Epigenetic regulation of gene expression plays a critical role in the development of liver cancer; however, the molecular mechanisms of epigenetic-driven liver cancers are not well understood. The aims of this study were to examine molecular mechanisms that cause the dedifferentiation of hepatocytes into cancer cells in aggressive hepatoblastoma and test if the inhibition of these mechanisms inhibits tumor growth.
Methods
We have analyzed CCAAT/Enhancer Binding Protein alpha (C/EBPα), Transcription factor Sp5, and histone deacetylase (HDAC)1 pathways from a large biobank of fresh hepatoblastoma (HBL) samples using high-pressure liquid chromatography–based examination of protein–protein complexes and have examined chromatin remodeling on the promoters of markers of hepatocytes and p21. The HDAC1 activity was inhibited in patient-derived xenograft models of HBL and in cultured hepatoblastoma cells and expression of HDAC1-dependent markers of hepatocytes was examined.
Results
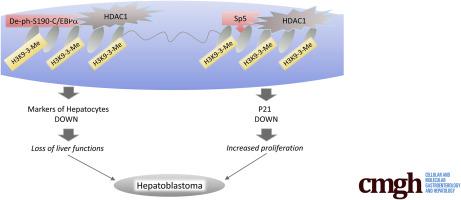
Analyses of a biobank showed that a significant portion of HBL patients have increased levels of an oncogenic de-phosphorylated-S190-C/EBPα, Sp5, and HDAC1 compared with amounts of these proteins in adjacent regions. We found that the oncogenic de-phosphorylated-S190-C/EBPα is created in aggressive HBL by protein phosphatase 2A, which is increased within the nucleus and dephosphorylates C/EBPα at Ser190. C/EBPα–HDAC1 and Sp5–HDAC1 complexes are abundant in hepatocytes, which dedifferentiate into cancer cells. Studies in HBL cells have shown that C/EBPα–HDAC1 and Sp5–HDAC1 complexes reduce markers of hepatocytes and p21 via repression of their promoters. Pharmacologic inhibition of C/EBPα–HDAC1 and Sp5–HDAC1 complexes by Suberoylanilide hydroxamic acid (SAHA) and small interfering RNA–mediated inhibition of HDAC1 increase expression of hepatocyte markers, p21, and inhibit proliferation of cancer cells.
Conclusions
HDAC1-mediated repression of markers of hepatocytes is an essential step for the development of HBL, providing background for generation of therapies for aggressive HBL by targeting HDAC1 activities.
中文翻译:

肝细胞标志物和 P21 的 HDAC1 依赖性抑制与小儿肝癌的发展有关
背景与目标
基因表达的表观遗传调控在肝癌的发生发展中起关键作用;然而,表观遗传驱动的肝癌的分子机制尚不清楚。本研究的目的是检查在侵袭性肝母细胞瘤中导致肝细胞去分化成癌细胞的分子机制,并测试这些机制的抑制是否会抑制肿瘤生长。
方法
我们使用基于高压液相色谱的蛋白质检查分析了来自新鲜肝母细胞瘤 (HBL) 样本的大型生物库中的 CCAAT/增强子结合蛋白 α (C/EBPα)、转录因子 Sp5 和组蛋白去乙酰化酶 (HDAC)1 途径——蛋白复合物,并检查了肝细胞标志物和 p21 的启动子上的染色质重塑。在患者衍生的 HBL 异种移植模型和培养的肝母细胞瘤细胞中,HDAC1 活性受到抑制,并检查了肝细胞的 HDAC1 依赖性标志物的表达。
结果
对生物库的分析表明,与相邻区域中这些蛋白质的含量相比,很大一部分 HBL 患者的致癌去磷酸化 S190-C/EBPα、Sp5 和 HDAC1 水平升高。我们发现致癌的去磷酸化-S190-C/EBPα 是由蛋白磷酸酶 2A 在侵袭性 HBL 中产生的,蛋白磷酸酶 2A 在细胞核内增加并在 Ser190 处使 C/EBPα 去磷酸化。C/EBPα-HDAC1 和 Sp5-HDAC1 复合物在肝细胞中含量丰富,可去分化为癌细胞。对 HBL 细胞的研究表明,C/EBPα-HDAC1 和 Sp5-HDAC1 复合物通过抑制其启动子来减少肝细胞和 p21 的标志物。
结论
HDAC1 介导的肝细胞标志物抑制是 HBL 发展的重要步骤,为通过靶向 HDAC1 活性产生侵袭性 HBL 疗法提供背景。

































 京公网安备 11010802027423号
京公网安备 11010802027423号