Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-07-07 , DOI: 10.1016/j.bioorg.2021.105151 Pradip K Gadekar 1 , Ganesh Urunkar 2 , Abhijit Roychowdhury 2 , Rajiv Sharma 2 , Julie Bose 3 , Smriti Khanna 4 , Anagha Damre 5 , S Sarveswari 6
|
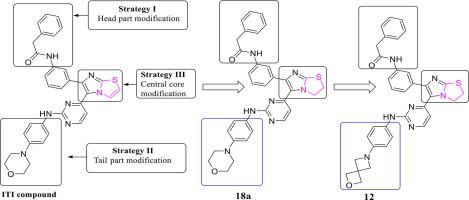
Herein we describe the design, synthesis and anticancer evaluation of a series of 2,3-dihydroimidazo[2,1-b]thiazoles as dual kinase inhibitors of IGF1R and EGFR. A series of saturated dihydroimidazo[2,1-b] thiazoles were synthesized to understand the structure–activity relationship.
Further, the key modifications were performed to improve drug like properties of the series. A 2-oxa-6-azaspiro [3.3] heptane moiety was incorporated as a bioisosteric replacement of morpholine on dihydroimidazo[2,1-b] thiazole scaffold.Subsequent structure–activity relationship (SAR) studies identified several compounds with nM range of activity. The compound 18a shows promising activity, IC50 = 52 nM against IGF1R and IC50 = 35.5 nM against EGFR with descent PK profile. The identified leadshows promising activity against both wild type and the T790M mutant forms of enzymes.
中文翻译:

2,3-二氢咪唑并[2,1-b]噻唑作为EGFR和IGF1R双重抑制剂的设计、合成和生物学评价
在此,我们描述了一系列 2,3-二氢咪唑并[2,1- b ]噻唑作为 IGF1R 和 EGFR 的双重激酶抑制剂的设计、合成和抗癌评估。合成了一系列饱和的二氢咪唑并[2,1-b] 噻唑以了解结构-活性关系。
此外,还进行了关键修改以改善该系列的类似药物的特性。将 2-oxa-6-azaspiro [3.3] 庚烷部分作为吗啉的生物等排替代物掺入二氢咪唑并 [2,1-b] 噻唑支架上。随后的结构-活性关系 (SAR) 研究确定了几种具有 nM 活性范围的化合物. 化合物18a显示出有希望的活性, 针对 IGF1R 的IC 50 = 52 nM 和 针对 EGFR 的IC 50 = 35.5 nM,具有下降 PK 特征。鉴定出的先导化合物对野生型和 T790M 突变型酶均具有良好的活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号