当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Atomic Insight into the Chemical Origin and Variation of the Dielectric Constant in Liquid Electrolytes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-05 , DOI: 10.1002/anie.202107657 Nan Yao 1 , Xiang Chen 1 , Xin Shen 1 , Rui Zhang 2 , Zhong-Heng Fu 1 , Xia-Xia Ma 1 , Xue-Qiang Zhang 1 , Bo-Quan Li 2 , Qiang Zhang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-07-05 , DOI: 10.1002/anie.202107657 Nan Yao 1 , Xiang Chen 1 , Xin Shen 1 , Rui Zhang 2 , Zhong-Heng Fu 1 , Xia-Xia Ma 1 , Xue-Qiang Zhang 1 , Bo-Quan Li 2 , Qiang Zhang 1
Affiliation
|
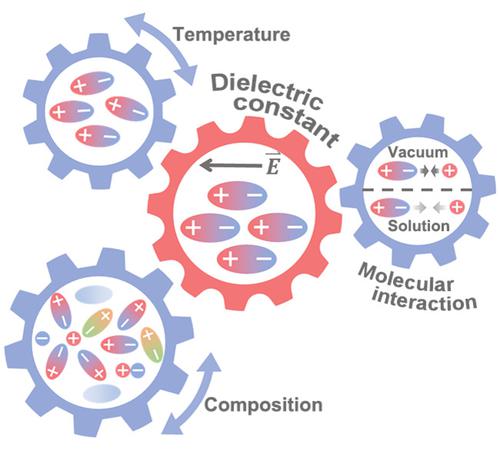
The dielectric constant is a crucial physicochemical property of liquids in tuning solute–solvent interactions and solvation microstructures. Herein the dielectric constant variation of liquid electrolytes regarding to temperatures and electrolyte compositions is probed by molecular dynamics simulations. Dielectric constants of solvents reduce as temperatures increase due to accelerated mobility of molecules. For solvent mixtures with different mixing ratios, their dielectric constants either follow a linear superposition rule or satisfy a polynomial function, depending on weak or strong intermolecular interactions. Dielectric constants of electrolytes exhibit a volcano trend with increasing salt concentrations, which can be attributed to dielectric contributions from salts and formation of solvation structures. This work affords an atomic insight into the dielectric constant variation and its chemical origin, which can deepen the fundamental understanding of solution chemistry.
中文翻译:

对液体电解质中介电常数的化学起源和变化的原子洞察
介电常数是液体在调节溶质 - 溶剂相互作用和溶剂化微观结构方面的关键物理化学性质。在本文中,液体电解质的介电常数随温度和电解质组成的变化是通过分子动力学模拟来探测的。由于分子的加速流动,溶剂的介电常数随着温度的升高而降低。对于具有不同混合比的溶剂混合物,它们的介电常数要么遵循线性叠加规则,要么满足多项式函数,这取决于分子间的弱相互作用或强相互作用。随着盐浓度的增加,电解质的介电常数呈现火山趋势,这可归因于盐的介电贡献和溶剂化结构的形成。
更新日期:2021-07-05
中文翻译:

对液体电解质中介电常数的化学起源和变化的原子洞察
介电常数是液体在调节溶质 - 溶剂相互作用和溶剂化微观结构方面的关键物理化学性质。在本文中,液体电解质的介电常数随温度和电解质组成的变化是通过分子动力学模拟来探测的。由于分子的加速流动,溶剂的介电常数随着温度的升高而降低。对于具有不同混合比的溶剂混合物,它们的介电常数要么遵循线性叠加规则,要么满足多项式函数,这取决于分子间的弱相互作用或强相互作用。随着盐浓度的增加,电解质的介电常数呈现火山趋势,这可归因于盐的介电贡献和溶剂化结构的形成。













































 京公网安备 11010802027423号
京公网安备 11010802027423号