当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tropospheric Oxidation of 1H-Heptafluorocyclopentene (cyc-CF2CF2CF2CF═CH−) with OH Radicals: Reaction Mechanism, Kinetics, and Global Warming Potentials
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-07-05 , DOI: 10.1021/acsearthspacechem.1c00124 Parineeta Gogoi 1 , Subrata Paul 2 , Bhupesh Kumar Mishra 3 , Nand Kishor Gour 1 , Ramesh Chandra Deka 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2021-07-05 , DOI: 10.1021/acsearthspacechem.1c00124 Parineeta Gogoi 1 , Subrata Paul 2 , Bhupesh Kumar Mishra 3 , Nand Kishor Gour 1 , Ramesh Chandra Deka 1
Affiliation
|
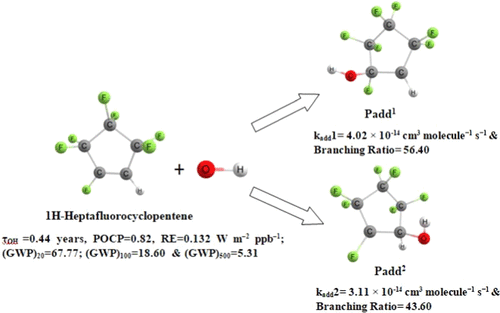
Assessment of the atmospheric chemistry and environmental impact of 1H-heptafluorocyclopentene (i.e., cyc-CF2CF2CF2CF═CH) appears essential before its wide-scale applications. So, in this present work, we have investigated the •OH radical-initiated oxidation of cyc-CF2CF2CF2CF═CH– using the density functional theory. We have used M11/6-311++G(d,p) level of theory for geometry optimization and frequency calculations. The energetics and rate constant calculations indicate that the pathway concerning the •OH radical addition reaction with the C═C bond is more favorable than the H- and F-abstraction reactions with the •OH radical. The canonical transition state theory has been employed to calculate the rate constant within the temperature range of 250–450 K and 1 atm pressure. The estimated overall rate constant value is 7.14 × 10–14 cm3 molecule–1 s–1 at 298.15 K, which agrees well with the previously reported experimental rate constant value of (5.20 ± 0.09) × 10–14 cm3 molecule–1 s–1 at 298.15 K. Moreover, we have determined the branching ratio percentage and found that the •OH radical addition reaction contributes 99.99% to the overall rate constant. The calculated atmospheric lifetime is 0.44 years. Furthermore, the radiative efficiency (RE) is determined to be 0.132 W m–2 ppb–1 and the photochemical ozone creation potential (POCPs) is also calculated as 0.82. Finally, global warming potentials (GWPs) for 20, 100, and 500 years are estimated as 67.77, 18.60, and 5.31, respectively.
中文翻译:

1的对流层氧化ħ -Heptafluorocyclopentene(CYC-CF 2 CF 2 CF 2反应机理,动力学,和全球变暖潜能:CF═CH-)与OH自由基
1 H-七氟环戊烯(即cyc-CF 2 CF 2 CF 2 CF= CH)的大气化学和环境影响评估在其大规模应用之前显得至关重要。因此,在目前的工作中,我们使用密度泛函理论研究了• OH 自由基引发的 cyc-CF 2 CF 2 CF 2 CF= CH–氧化。我们已使用 M11/6-311++G(d,p) 理论水平进行几何优化和频率计算。能量学和速率常数计算表明,关于•与 C=C 键的 OH 自由基加成反应比与• OH 自由基的 H-和 F-抽象反应更有利。已采用规范过渡态理论来计算 250-450 K 和 1 个大气压压力范围内的速率常数。估计的总速率常数值为 7.14 × 10 –14 cm 3分子–1 s –1在 298.15 K,这与先前报道的实验速率常数值 (5.20 ± 0.09) × 10 –14 cm 3分子–1 一致s –1在 298.15 K。此外,我们已经确定了支化率百分比,发现•OH自由基加成反应对总速率常数贡献了99.99%。计算出的大气寿命为 0.44 年。此外,辐射效率 (RE) 确定为 0.132 W m –2 ppb –1,光化学臭氧产生潜能 (POCP) 也计算为 0.82。最后,20、100 和 500 年的全球变暖潜能值 (GWP) 分别估计为 67.77、18.60 和 5.31。
更新日期:2021-07-15
中文翻译:

1的对流层氧化ħ -Heptafluorocyclopentene(CYC-CF 2 CF 2 CF 2反应机理,动力学,和全球变暖潜能:CF═CH-)与OH自由基
1 H-七氟环戊烯(即cyc-CF 2 CF 2 CF 2 CF= CH)的大气化学和环境影响评估在其大规模应用之前显得至关重要。因此,在目前的工作中,我们使用密度泛函理论研究了• OH 自由基引发的 cyc-CF 2 CF 2 CF 2 CF= CH–氧化。我们已使用 M11/6-311++G(d,p) 理论水平进行几何优化和频率计算。能量学和速率常数计算表明,关于•与 C=C 键的 OH 自由基加成反应比与• OH 自由基的 H-和 F-抽象反应更有利。已采用规范过渡态理论来计算 250-450 K 和 1 个大气压压力范围内的速率常数。估计的总速率常数值为 7.14 × 10 –14 cm 3分子–1 s –1在 298.15 K,这与先前报道的实验速率常数值 (5.20 ± 0.09) × 10 –14 cm 3分子–1 一致s –1在 298.15 K。此外,我们已经确定了支化率百分比,发现•OH自由基加成反应对总速率常数贡献了99.99%。计算出的大气寿命为 0.44 年。此外,辐射效率 (RE) 确定为 0.132 W m –2 ppb –1,光化学臭氧产生潜能 (POCP) 也计算为 0.82。最后,20、100 和 500 年的全球变暖潜能值 (GWP) 分别估计为 67.77、18.60 和 5.31。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号