当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BF3·Et2O as a metal-free catalyst for direct reductive amination of aldehydes with amines using formic acid as a reductant
Green Chemistry ( IF 9.3 ) Pub Date : 2021-06-17 , DOI: 10.1039/d1gc01468d
Zhenli Luo 1, 2, 3, 4 , Yixiao Pan 1, 2, 3, 4, 5 , Zhen Yao 1, 2, 3, 4 , Ji Yang 1, 2, 3, 4 , Xin Zhang 1, 2, 3, 4 , Xintong Liu 1, 2, 3, 4 , Lijin Xu 1, 2, 3, 4 , Qing-Hua Fan 5, 6, 7, 8
Green Chemistry ( IF 9.3 ) Pub Date : 2021-06-17 , DOI: 10.1039/d1gc01468d
Zhenli Luo 1, 2, 3, 4 , Yixiao Pan 1, 2, 3, 4, 5 , Zhen Yao 1, 2, 3, 4 , Ji Yang 1, 2, 3, 4 , Xin Zhang 1, 2, 3, 4 , Xintong Liu 1, 2, 3, 4 , Lijin Xu 1, 2, 3, 4 , Qing-Hua Fan 5, 6, 7, 8
Affiliation
|
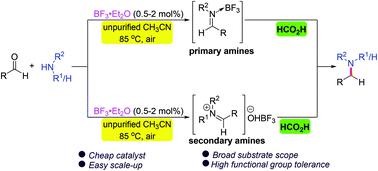
A versatile metal- and base-free direct reductive amination of aldehydes with amines using formic acid as a reductant under the catalysis of inexpensive BF3·Et2O has been developed. A wide range of primary and secondary amines and diversely substituted aldehydes are compatible with this transformation, allowing facile access to various secondary and tertiary amines in high yields with wide functional group tolerance. Moreover, the method is convenient for the late-stage functionalization of bioactive compounds and preparation of commercialized drug molecules and biologically relevant N-heterocycles. The procedure has the advantages of simple operation and workup and easy scale-up, and does not require dry conditions, an inert atmosphere or a water scavenger. Mechanistic studies reveal the involvement of imine activation by BF3 and hydride transfer from formic acid.
中文翻译:

BF3·Et2O作为无金属催化剂,用于以甲酸为还原剂的醛与胺的直接还原胺化
在廉价的 BF 3 ·Et 2催化下,使用甲酸作为还原剂的醛与胺的通用无金属和无碱直接还原胺化O 已开发。广泛的伯胺和仲胺以及不同取代的醛与这种转化相容,从而可以轻松获得各种仲胺和叔胺,高产率和广泛的官能团耐受性。此外,该方法有利于生物活性化合物的后期功能化以及商业化药物分子和生物相关氮杂环的制备。该工艺操作简单,易于放大,不需要干燥条件、惰性气氛或除水剂。机理研究揭示了 BF 3对亚胺的活化和甲酸的氢化物转移的参与。
更新日期:2021-07-06
中文翻译:

BF3·Et2O作为无金属催化剂,用于以甲酸为还原剂的醛与胺的直接还原胺化
在廉价的 BF 3 ·Et 2催化下,使用甲酸作为还原剂的醛与胺的通用无金属和无碱直接还原胺化O 已开发。广泛的伯胺和仲胺以及不同取代的醛与这种转化相容,从而可以轻松获得各种仲胺和叔胺,高产率和广泛的官能团耐受性。此外,该方法有利于生物活性化合物的后期功能化以及商业化药物分子和生物相关氮杂环的制备。该工艺操作简单,易于放大,不需要干燥条件、惰性气氛或除水剂。机理研究揭示了 BF 3对亚胺的活化和甲酸的氢化物转移的参与。

































 京公网安备 11010802027423号
京公网安备 11010802027423号