Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2021-07-03 , DOI: 10.1016/j.jmb.2021.167145 Noah J Harris 1 , Meredith L Jenkins 1 , Udit Dalwadi 2 , Kaelin D Fleming 1 , Sung-Eun Nam 2 , Matthew A H Parson 1 , Calvin K Yip 2 , John E Burke 3
|
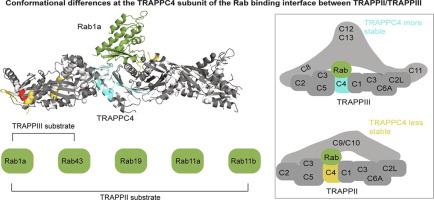
Transport Protein Particle complexes (TRAPP) are evolutionarily conserved regulators of membrane trafficking, with this mediated by their guanine nucleotide exchange factor (GEF) activity towards Rab GTPases. In metazoans evidence suggests that two different TRAPP complexes exist, TRAPPII and TRAPPIII. These two complexes share a common core of subunits, with complex specific subunits (TRAPPC9 and TRAPPC10 in TRAPPII and TRAPPC8, TRAPPC11, TRAPPC12, TRAPPC13 in TRAPPIII). TRAPPII and TRAPPIII have distinct specificity for GEF activity towards Rabs, with TRAPPIII acting on Rab1, and TRAPPII acting on Rab1 and Rab11. The molecular basis for how these complex specific subunits alter GEF activity towards Rab GTPases is unknown. Here we have used a combination of biochemical assays, hydrogen deuterium exchange mass spectrometry (HDX-MS) and electron microscopy to examine the regulation of TRAPPII and TRAPPIIII complexes in solution and on membranes. GEF assays revealed that TRAPPIII has GEF activity against Rab1 and Rab43, with no detectable activity against the other 18 Rabs tested. The TRAPPIII complex had significant differences in protein dynamics at the Rab binding site compared to TRAPPII, potentially indicating an important role of accessory subunits in altering the active site of TRAPP complexes. Both the TRAPPII and TRAPPIII complexes had enhanced GEF activity on lipid membranes, with HDX-MS revealing numerous conformational changes that accompany membrane association. HDX-MS also identified a membrane binding site in TRAPPC8. Collectively, our results provide insight into the functions of TRAPP complexes and how they can achieve Rab specificity.
中文翻译:

哺乳动物 TRAPPIII 复合物新型 Rab-GEF 活性的生化洞察
转运蛋白粒子复合物 (TRAPP) 是膜运输的进化保守调节剂,这是由它们对 Rab GTPases 的鸟嘌呤核苷酸交换因子 (GEF) 活性介导的。在后生动物中,证据表明存在两种不同的 TRAPP 复合体,即 TRAPPII 和 TRAPPIII。这两个复合物共享一个共同的亚基核心,具有复杂的特定亚基(TRAPPC9 和 TRAPPC10 在 TRAPPII 和 TRAPPC8、TRAPPC11、TRAPPC12、TRAPPC13 在 TRAPPC13)。TRAPPII 和 TRAPPIII 对 GEF 对 Rab 的活性具有明显的特异性,TRAPPIII 作用于 Rab1,而 TRAPPII 作用于 Rab1 和 Rab11。这些复杂的特定亚基如何改变 GEF 对 Rab GTP 酶的活性的分子基础尚不清楚。在这里,我们使用了生化分析的组合,氢氘交换质谱 (HDX-MS) 和电子显微镜检查 TRAPPII 和 TRAPPIIII 复合物在溶液和膜上的调节。GEF 测定显示 TRAPPIII 具有针对 Rab1 和 Rab43 的 GEF 活性,对其他 18 个测试的 Rab 没有可检测的活性。与 TRAPPII 相比,TRAPPIII 复合物在 Rab 结合位点的蛋白质动力学方面具有显着差异,这可能表明辅助亚基在改变 TRAPP 复合物的活性位点中的重要作用。TRAPPII 和 TRAPPIII 复合物都增强了 GEF 对脂质膜的活性,HDX-MS 揭示了伴随膜结合的许多构象变化。HDX-MS 还鉴定了 TRAPPC8 中的膜结合位点。总的来说,















































 京公网安备 11010802027423号
京公网安备 11010802027423号