当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Roles of the Electrode/Electrolyte Interface for Enabling Stable Li∥Sulfurized Polyacrylonitrile Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-07-02 , DOI: 10.1021/acsami.1c07903
Zhaohui Wu 1 , Seong-Min Bak 2 , Zulipiya Shadike 3 , Sicen Yu 4 , Enyuan Hu 3 , Xing Xing 4 , Yonghua Du 2 , Xiao-Qing Yang 3 , Haodong Liu 1, 5 , Ping Liu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-07-02 , DOI: 10.1021/acsami.1c07903
Zhaohui Wu 1 , Seong-Min Bak 2 , Zulipiya Shadike 3 , Sicen Yu 4 , Enyuan Hu 3 , Xing Xing 4 , Yonghua Du 2 , Xiao-Qing Yang 3 , Haodong Liu 1, 5 , Ping Liu 1
Affiliation
|
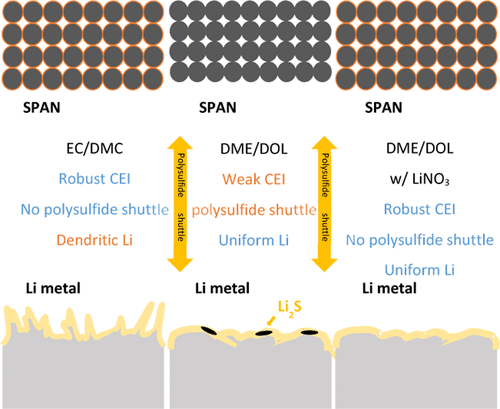
Sulfurized polyacrylonitrile (SPAN) is a promising high-capacity cathode material. In this work, we use spatially resolved X-ray absorption spectroscopy combined with X-ray fluorescence (XRF) microscopy, X-ray photoelectron spectroscopy, and scanning electron microscopy to examine the structural transformation of SPAN and the critical role of a robust cathode–electrolyte interface (CEI) on the electrode. LiSx species forms during the cycling of SPAN. However, in carbonate-based electrolytes and ether-based electrolytes with LiNO3 additives, these species are well protected by the CEI and do not dissolve into the electrolytes. In contrast, in an ether-based electrolyte without the LiNO3 additive, LiSx species dissolve into the electrolyte, resulting in the shuttle effect and capacity loss. Examination of the Li anode by XRF and SEM reveals dense spherical Li morphology in ether-based electrolytes, but sulfur is present in the absence of the LiNO3 additive. In contrast, porous dendritic Li is found in the carbonate electrolyte. These analyses established that an ether-based electrolyte with LiNO3 is a superior choice that enables stable cycling of both electrodes. Based on these insights, we successfully demonstrate the stable cycling of high areal loading SPAN cathode (>6.5 mA h cm–2) with lean electrolyte amounts, showing promising Li∥SPAN cell performance under practical conditions.
中文翻译:

了解电极/电解质界面在实现稳定的锂∥硫化聚丙烯腈电池中的作用
硫化聚丙烯腈(SPAN)是一种很有前途的高容量正极材料。在这项工作中,我们使用空间分辨 X 射线吸收光谱结合 X 射线荧光 (XRF) 显微镜、X 射线光电子能谱和扫描电子显微镜来检查 SPAN 的结构转变和稳健阴极的关键作用——电极上的电解质界面 (CEI)。LiS x物种在 SPAN 循环过程中形成。然而,在含有 LiNO 3添加剂的碳酸盐基电解质和醚基电解质中,这些物质受到 CEI 的良好保护并且不会溶解到电解质中。相比之下,在没有 LiNO 3添加剂的醚基电解质中,LiS x物质溶解到电解质中,导致穿梭效应和容量损失。通过 XRF 和 SEM 对锂负极的检查揭示了醚基电解质中致密的球形锂形态,但在没有 LiNO 3添加剂的情况下存在硫。相反,在碳酸盐电解质中发现了多孔树枝状锂。这些分析表明,具有 LiNO 3的醚基电解质是一种卓越的选择,可以实现两个电极的稳定循环。基于这些见解,我们成功地证明了高面积负载 SPAN 正极 (>6.5 mA h cm –2 ) 和稀电解质量的稳定循环,在实际条件下显示出有希望的 Li∥SPAN 电池性能。
更新日期:2021-07-14
中文翻译:

了解电极/电解质界面在实现稳定的锂∥硫化聚丙烯腈电池中的作用
硫化聚丙烯腈(SPAN)是一种很有前途的高容量正极材料。在这项工作中,我们使用空间分辨 X 射线吸收光谱结合 X 射线荧光 (XRF) 显微镜、X 射线光电子能谱和扫描电子显微镜来检查 SPAN 的结构转变和稳健阴极的关键作用——电极上的电解质界面 (CEI)。LiS x物种在 SPAN 循环过程中形成。然而,在含有 LiNO 3添加剂的碳酸盐基电解质和醚基电解质中,这些物质受到 CEI 的良好保护并且不会溶解到电解质中。相比之下,在没有 LiNO 3添加剂的醚基电解质中,LiS x物质溶解到电解质中,导致穿梭效应和容量损失。通过 XRF 和 SEM 对锂负极的检查揭示了醚基电解质中致密的球形锂形态,但在没有 LiNO 3添加剂的情况下存在硫。相反,在碳酸盐电解质中发现了多孔树枝状锂。这些分析表明,具有 LiNO 3的醚基电解质是一种卓越的选择,可以实现两个电极的稳定循环。基于这些见解,我们成功地证明了高面积负载 SPAN 正极 (>6.5 mA h cm –2 ) 和稀电解质量的稳定循环,在实际条件下显示出有希望的 Li∥SPAN 电池性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号