Nano Research ( IF 9.5 ) Pub Date : 2021-07-01 , DOI: 10.1007/s12274-021-3665-8 Shi He , Vasishta Somayaji , Mengdi Wang , Seung-Hoon Lee , Zhijia Geng , Siyuan Zhu , Peter Novello , Chakrapani V. Varanasi , Jie Liu
|
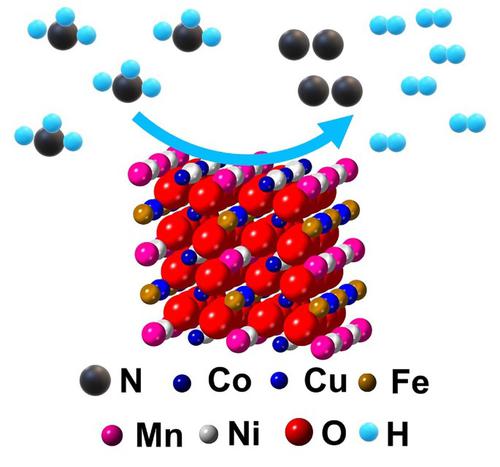
Ammonia has emerged as a promising energy carrier owing to its carbon neutral content and low expense in long-range transportation. Therefore, development of a specific pathway to release the energy stored in ammonia is therefore in urgent demand. Electrochemical oxidation provides a convenient and reliable route to attain efficient utilization of ammonia. Here, we report that the high entropy (Mn, Fe, Co, Ni, Cu)3O4 oxides can achieve high electrocatalytic activity for ammonia oxidation reaction (AOR) in non-aqueous solutions. The AOR onset overpotential of (Mn, Fe, Co, Ni, Cu)3O4 is 0.70 V, which is nearly 0.2 V lower than that of their most active single metal cation counterpart. The mass spectroscopy study reveals that (Mn, Fe, Co, Ni, Cu)3O4 preferentially oxidizes ammonia to environmentally friendly diatomic nitrogen with a Faradic efficiency of over 85%. The X-ray photoelectron spectroscopy (XPS) result indicates that the balancing metal d-band of Mn and Cu cations helps retain a long-lasting electrocatalytic activity. Overall, this work introduces a new family of earth-abundant transition metal high entropy oxide electrocatalysts for AOR, thus heralding a new paradigm of catalyst design for enabling ammonia as an energy carrier.
中文翻译:

用于氨的高效电化学氧化的高熵尖晶石氧化物
由于其碳中性含量和远程运输成本低,氨已成为一种有前途的能源载体。因此,迫切需要开发一种特定途径来释放储存在氨中的能量。电化学氧化提供了一种方便可靠的途径来实现氨的有效利用。在这里,我们报告了高熵 (Mn、Fe、Co、Ni、Cu) 3 O 4氧化物可以在非水溶液中实现氨氧化反应 (AOR) 的高电催化活性。(Mn, Fe, Co, Ni, Cu) 3 O 4的AOR起始过电位为 0.70 V,比其最活跃的单金属阳离子对应物低近 0.2 V。质谱研究表明,(Mn, Fe, Co, Ni, Cu) 3 O 4优先将氨氧化为环境友好的双原子氮,法拉第效率超过85%。X 射线光电子能谱 (XPS) 结果表明,Mn 和 Cu 阳离子的平衡金属 d 带有助于保持持久的电催化活性。总的来说,这项工作为 AOR 引入了一个新的地球丰富的过渡金属高熵氧化物电催化剂家族,从而预示着一种新的催化剂设计范式,使氨作为能量载体。































 京公网安备 11010802027423号
京公网安备 11010802027423号