当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the Intermediate Reaction Complexes and Critical Role of Support-Derived Oxygen Atoms in CO Oxidation on Single-Atom Pt/CeO2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-07-01 , DOI: 10.1021/acscatal.1c01900 Yubing Lu 1 , Shulan Zhou 2, 3 , Chun-Te Kuo 1 , Deepak Kunwar 4 , Coogan Thompson 1 , Adam S. Hoffman 5 , Alexey Boubnov 5 , Sen Lin 2, 6 , Abhaya K. Datye 4 , Hua Guo 2 , Ayman M. Karim 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-07-01 , DOI: 10.1021/acscatal.1c01900 Yubing Lu 1 , Shulan Zhou 2, 3 , Chun-Te Kuo 1 , Deepak Kunwar 4 , Coogan Thompson 1 , Adam S. Hoffman 5 , Alexey Boubnov 5 , Sen Lin 2, 6 , Abhaya K. Datye 4 , Hua Guo 2 , Ayman M. Karim 1
Affiliation
|
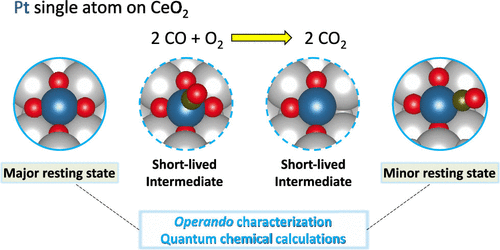
CeO2-supported Pt single-atom catalysts have been extensively studied due to their relevance in automobile emission control and for the fundamental understanding of CeO2-based catalysts. Though CeO2-supported Pt nanoparticles are often more active than their single-atom counterparts, the former could easily redisperse to Pt single atom under oxidizing diesel conditions. Therefore, to maximize the reactivity of every Pt atom, it is important to fully understand the reaction mechanism of CeO2-supported Pt single atoms. Here, we report a CO oxidation study on a Pt/CeO2 single-atom catalyst, where we can account for all of the neighbors using in situ and operando spectroscopy techniques and microcalorimetric measurements. Coupled with density functional theory calculations, we present a comprehensive picture of the dynamics of the surface species, the role of surface intermediates, and explain the observed reaction kinetics. We started with a catalyst containing exclusively single atoms and used in situ/operando spectroscopy to provide evidence for their stability during the reaction and to identify the Pt1 complexes before and during the reaction and their binding to CO. The results reveal that in the precatalyst, Pt is present as Pt(O)4 on the CeO2(111) step edge sites, but during CO oxidation, we find that two Pt1 complexes coexist, representing two states of the same active site in the reaction cycle. The dominant state/complex remains Pt(O)4, which adsorbs CO very weakly as shown by CO microcalorimetry. The second, minority state/complex, Pt(CO)(O)3 is generated through the reaction of Pt(O)4 with CO, and CO is bound strongly to Pt1. Labile oxygen adatoms from the CeO2 surface play a major role in the regeneration of Pt(O)4 either directly from Pt(O)3 or by reaction with the strongly adsorbed CO in Pt(CO)(O)3. We show that the formation of an oxygen vacancy and generation of a labile O* are not barrierless, which explains the long lifetime of Pt(CO)(O)3 and its detectability despite being a minority complex. The results help to develop a comprehensive view of the dynamic evolution of Pt1 complexes along the reaction cycle and provide mechanistic insights to guide the design of Pt-based single-atom catalysts.
中文翻译:

解开中间反应配合物和支持衍生的氧原子在单原子 Pt/CeO 2上的 CO 氧化中的关键作用
CeO 2负载的Pt单原子催化剂由于其在汽车排放控制中的相关性以及对基于CeO 2的催化剂的基本理解而被广泛研究。尽管 CeO 2负载的 Pt 纳米粒子通常比它们的单原子对应物更具活性,但前者在氧化柴油条件下很容易重新分散为 Pt 单原子。因此,为了最大限度地提高每个 Pt 原子的反应性,充分了解 CeO 2负载的 Pt 单原子的反应机理非常重要。在这里,我们报告了关于 Pt/CeO 2单原子催化剂的 CO 氧化研究,我们可以在其中解释使用原位和操作数的所有邻居光谱技术和微量热测量。结合密度泛函理论计算,我们全面展示了表面物种的动力学、表面中间体的作用,并解释了观察到的反应动力学。我们从仅包含单个原子的催化剂开始,并使用原位/原位光谱学来提供它们在反应过程中的稳定性的证据,并在反应之前和期间鉴定 Pt 1配合物以及它们与 CO 的结合。结果表明,在预催化剂中, Pt 以 Pt(O) 4 的形式存在于 CeO 2 (111) 台阶边缘位置,但在 CO 氧化过程中,我们发现两个 Pt 1配合物共存,代表反应循环中同一活性位点的两种状态。占主导地位的状态/配合物仍然是 Pt(O) 4,它非常微弱地吸附 CO,如 CO 微量热法所示。第二种少数态/络合物 Pt(CO)(O) 3是通过 Pt(O) 4与 CO的反应生成的,并且 CO 与 Pt 1紧密结合。CeO 2表面的不稳定氧吸附原子在直接从 Pt(O) 3或通过与 Pt(CO)(O) 3 中强吸附的 CO 反应的 Pt(O) 4再生中起主要作用. 我们表明,氧空位的形成和不稳定的 O* 的产生不是无障碍的,这解释了 Pt(CO)(O) 3的长寿命及其尽管是少数复合物的可检测性。该结果有助于全面了解 Pt 1配合物在反应循环中的动态演变,并为指导 Pt 基单原子催化剂的设计提供机理见解。
更新日期:2021-07-16
中文翻译:

解开中间反应配合物和支持衍生的氧原子在单原子 Pt/CeO 2上的 CO 氧化中的关键作用
CeO 2负载的Pt单原子催化剂由于其在汽车排放控制中的相关性以及对基于CeO 2的催化剂的基本理解而被广泛研究。尽管 CeO 2负载的 Pt 纳米粒子通常比它们的单原子对应物更具活性,但前者在氧化柴油条件下很容易重新分散为 Pt 单原子。因此,为了最大限度地提高每个 Pt 原子的反应性,充分了解 CeO 2负载的 Pt 单原子的反应机理非常重要。在这里,我们报告了关于 Pt/CeO 2单原子催化剂的 CO 氧化研究,我们可以在其中解释使用原位和操作数的所有邻居光谱技术和微量热测量。结合密度泛函理论计算,我们全面展示了表面物种的动力学、表面中间体的作用,并解释了观察到的反应动力学。我们从仅包含单个原子的催化剂开始,并使用原位/原位光谱学来提供它们在反应过程中的稳定性的证据,并在反应之前和期间鉴定 Pt 1配合物以及它们与 CO 的结合。结果表明,在预催化剂中, Pt 以 Pt(O) 4 的形式存在于 CeO 2 (111) 台阶边缘位置,但在 CO 氧化过程中,我们发现两个 Pt 1配合物共存,代表反应循环中同一活性位点的两种状态。占主导地位的状态/配合物仍然是 Pt(O) 4,它非常微弱地吸附 CO,如 CO 微量热法所示。第二种少数态/络合物 Pt(CO)(O) 3是通过 Pt(O) 4与 CO的反应生成的,并且 CO 与 Pt 1紧密结合。CeO 2表面的不稳定氧吸附原子在直接从 Pt(O) 3或通过与 Pt(CO)(O) 3 中强吸附的 CO 反应的 Pt(O) 4再生中起主要作用. 我们表明,氧空位的形成和不稳定的 O* 的产生不是无障碍的,这解释了 Pt(CO)(O) 3的长寿命及其尽管是少数复合物的可检测性。该结果有助于全面了解 Pt 1配合物在反应循环中的动态演变,并为指导 Pt 基单原子催化剂的设计提供机理见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号