当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decomposition of Trace Li2CO3 During Charging Leads to Cathode Interface Degradation with the Solid Electrolyte LLZO
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-07-01 , DOI: 10.1002/adfm.202103716 Alexander A. Delluva 1, 2 , Jonas Kulberg‐Savercool 1 , Adam Holewinski 1, 2, 3
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-07-01 , DOI: 10.1002/adfm.202103716 Alexander A. Delluva 1, 2 , Jonas Kulberg‐Savercool 1 , Adam Holewinski 1, 2, 3
Affiliation
|
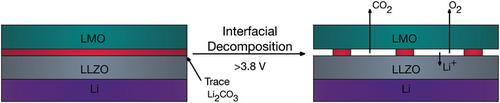
A major challenge for lithium-containing electrochemical systems is the formation of lithium carbonates. Solid-state electrolytes circumvent the use of organic liquids that can generate these species, but they are still susceptible to Li2CO3 formation from exposure to water vapor and carbon dioxide. It is reported here that trace quantities of Li2CO3, which are re-formed following standard mitigation and handling procedures, can decompose at high charging potentials and degrade the electrolyte–cathode interface. Operando electrochemical mass spectrometry (EC–MS) is employed to monitor the outgassing of solid-state batteries containing the garnet electrolyte Li7La3Zr2O12 (LLZO) and using appropriate controls CO2 and O2 are identified to emanate from the electrolyte–cathode interface at charging potentials > 3.8 V (vs Li/Li+). The gas evolution is correlated with a large increase in cathode interfacial resistance observed by potential-resolved impedance spectroscopy. This is the first evidence of electrochemical decomposition of interfacial Li2CO3 in garnet cells and suggests a need to report “time-to-assembly” for cell preparation methods.
中文翻译:

充电过程中痕量 Li2CO3 的分解导致与固体电解质 LLZO 的阴极界面退化
含锂电化学系统的主要挑战是碳酸锂的形成。固态电解质避免使用可产生这些物质的有机液体,但它们仍然容易因暴露于水蒸气和二氧化碳而形成Li 2 CO 3。据报道,微量的 Li 2 CO 3按照标准的缓解和处理程序重新形成,可以在高充电电位下分解并降解电解质-阴极界面。使用操作电化学质谱 (EC-MS) 监测含有石榴石电解质 Li 7 La 3 Zr 2的固态电池的除气O 12 (LLZO) 和使用适当的控制CO 2和O 2被确定为在充电电位> 3.8 V(相对于Li/Li +)时从电解质-阴极界面发出。气体逸出与通过电位分辨阻抗谱观察到的阴极界面电阻的大幅增加相关。这是石榴石电池中界面 Li 2 CO 3电化学分解的第一个证据,并表明需要报告电池制备方法的“组装时间”。
更新日期:2021-08-20
中文翻译:

充电过程中痕量 Li2CO3 的分解导致与固体电解质 LLZO 的阴极界面退化
含锂电化学系统的主要挑战是碳酸锂的形成。固态电解质避免使用可产生这些物质的有机液体,但它们仍然容易因暴露于水蒸气和二氧化碳而形成Li 2 CO 3。据报道,微量的 Li 2 CO 3按照标准的缓解和处理程序重新形成,可以在高充电电位下分解并降解电解质-阴极界面。使用操作电化学质谱 (EC-MS) 监测含有石榴石电解质 Li 7 La 3 Zr 2的固态电池的除气O 12 (LLZO) 和使用适当的控制CO 2和O 2被确定为在充电电位> 3.8 V(相对于Li/Li +)时从电解质-阴极界面发出。气体逸出与通过电位分辨阻抗谱观察到的阴极界面电阻的大幅增加相关。这是石榴石电池中界面 Li 2 CO 3电化学分解的第一个证据,并表明需要报告电池制备方法的“组装时间”。






























 京公网安备 11010802027423号
京公网安备 11010802027423号