当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aryl carbazole-based macrocycles: synthesis, their remarkably stable radical cations and host–guest complexation with fullerenes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-06-22 , DOI: 10.1039/d1qo00686j Lijun Mao 1 , Manfei Zhou 1 , Yan-Fei Niu 1 , Xiao-Li Zhao 1 , Xueliang Shi 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-06-22 , DOI: 10.1039/d1qo00686j Lijun Mao 1 , Manfei Zhou 1 , Yan-Fei Niu 1 , Xiao-Li Zhao 1 , Xueliang Shi 1
Affiliation
|
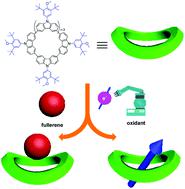
A series of fully conjugated macrocycles Mn (n = 4–7) consisting of N-(3,5-di-tert-butyl-4-methoxyphenyl) substituted carbazole (Cz-Ar) were successfully synthesized. The aryl carbazole and macrocycle M4 can be readily oxidized and the corresponding radical cation species were found to be highly stable. Moreover, macrocycle M5 was found to form 1 : 1 stoichiometric complexes with fullerenes C60 and C70 with association constants as high as (8.38 ± 0.33) × 104 M−1 and (7.64 ± 0.26) × 104 M−1, respectively.
中文翻译:

基于芳基咔唑的大环化合物:合成、它们非常稳定的自由基阳离子以及与富勒烯的主客体络合
成功合成了一系列由N -(3,5-二叔丁基-4-甲氧基苯基)取代的咔唑 ( Cz-Ar )组成的完全共轭大环M n ( n = 4–7) 。芳基咔唑和大环M 4很容易被氧化,并且发现相应的自由基阳离子物质是高度稳定的。此外,发现大环M 5与富勒烯 C 60和 C 70形成 1 : 1 化学计量配合物,结合常数高达 (8.38 ± 0.33) × 10 4 M -1和 (7.64 ± 0.26) × 10 4 M-1,分别。
更新日期:2021-06-30
中文翻译:

基于芳基咔唑的大环化合物:合成、它们非常稳定的自由基阳离子以及与富勒烯的主客体络合
成功合成了一系列由N -(3,5-二叔丁基-4-甲氧基苯基)取代的咔唑 ( Cz-Ar )组成的完全共轭大环M n ( n = 4–7) 。芳基咔唑和大环M 4很容易被氧化,并且发现相应的自由基阳离子物质是高度稳定的。此外,发现大环M 5与富勒烯 C 60和 C 70形成 1 : 1 化学计量配合物,结合常数高达 (8.38 ± 0.33) × 10 4 M -1和 (7.64 ± 0.26) × 10 4 M-1,分别。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号