当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Stereoselective and Scalable Synthesis for the Potent Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor, BMT-297376; N-((R)-1-((cis)-4-(3-(Difluoromethyl)-2-methoxypyridin-4-yl)cyclohexyl)propyl)-6-methoxynicotinamide
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.oprd.1c00142 Roshan Y. Nimje 1 , Prakasam Kuppusamy 1 , Suresh Krishnamoorthy 1 , Yoganand Shanmugam 1 , Duraisamy Ramasamy 1 , Haridhas Manoharan 1 , Pirama Nayagam Arunachalam 1 , Aaron Balog 2 , Emily C. Cherney 2 , Liping Zhang 2 , Robert M. Borzilleri 2 , Zhenqiu Hong 2 , James Kempson 2 , Richard R. Rampulla 2 , Arvind Mathur 2 , Anuradha Gupta 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.oprd.1c00142 Roshan Y. Nimje 1 , Prakasam Kuppusamy 1 , Suresh Krishnamoorthy 1 , Yoganand Shanmugam 1 , Duraisamy Ramasamy 1 , Haridhas Manoharan 1 , Pirama Nayagam Arunachalam 1 , Aaron Balog 2 , Emily C. Cherney 2 , Liping Zhang 2 , Robert M. Borzilleri 2 , Zhenqiu Hong 2 , James Kempson 2 , Richard R. Rampulla 2 , Arvind Mathur 2 , Anuradha Gupta 1
Affiliation
|
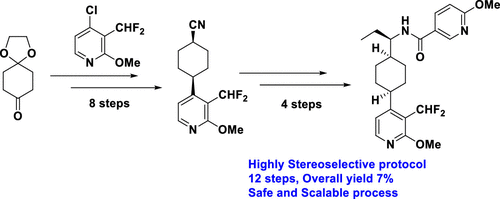
The current work describes a stereoselective and scalable route to N-((R)-1-((cis)-4-(3-(difluoromethyl)-2-methoxypyridin-4-yl)cyclohexyl)propyl)-6-methoxynicotinamide (1) from readily available 1,4-dioxaspiro[4.5]decan-8-one. The developed process encompasses an efficient 1,4-trans-selective synthesis of (trans)-4-(3-(difluoromethyl)-2-methoxypyridin-4-yl)cyclohexyl methanesulfonate as the key intermediate and the use of Ellman sulfinamine methodology to install an alkyl amine in a stereoselective manner. Various synthetic routes were screened to accomplish a stereoselective and scalable protocol to access the title compound (1). This advancement enabled a competent route to the title compound in an enantioselective, safe, cost-effective, and scalable manner.
中文翻译:

开发强效吲哚胺 2,3-双加氧酶 1 (IDO1) 抑制剂 BMT-297376 的立体选择性和可扩展合成;N -(( R )-1-((顺式)-4-(3-(二氟甲基)-2-甲氧基吡啶-4-基)环己基)丙基)-6-甲氧基烟酰胺
目前的工作描述了一种立体选择性和可扩展的路线,用于N -(( R )-1-((顺式)-4-(3-(二氟甲基)-2-甲氧基吡啶-4-基)环己基)丙基)-6-甲氧基烟酰胺 ( 1 ) 来自容易获得的 1,4-二氧杂螺[4.5] decan-8-one。开发的工艺包括(反式)-4-(3-(二氟甲基)-2-甲氧基吡啶-4-基)环己基甲磺酸酯的有效 1,4-反式选择性合成作为关键中间体,并使用 Ellman 亚磺胺方法以立体选择性方式安装烷基胺。筛选了各种合成路线以实现立体选择性和可扩展的协议来访问标题化合物 ( 1)。这一进步使得以对映选择性、安全、经济高效和可扩展的方式获得标题化合物的有效途径成为可能。
更新日期:2021-07-16
中文翻译:

开发强效吲哚胺 2,3-双加氧酶 1 (IDO1) 抑制剂 BMT-297376 的立体选择性和可扩展合成;N -(( R )-1-((顺式)-4-(3-(二氟甲基)-2-甲氧基吡啶-4-基)环己基)丙基)-6-甲氧基烟酰胺
目前的工作描述了一种立体选择性和可扩展的路线,用于N -(( R )-1-((顺式)-4-(3-(二氟甲基)-2-甲氧基吡啶-4-基)环己基)丙基)-6-甲氧基烟酰胺 ( 1 ) 来自容易获得的 1,4-二氧杂螺[4.5] decan-8-one。开发的工艺包括(反式)-4-(3-(二氟甲基)-2-甲氧基吡啶-4-基)环己基甲磺酸酯的有效 1,4-反式选择性合成作为关键中间体,并使用 Ellman 亚磺胺方法以立体选择性方式安装烷基胺。筛选了各种合成路线以实现立体选择性和可扩展的协议来访问标题化合物 ( 1)。这一进步使得以对映选择性、安全、经济高效和可扩展的方式获得标题化合物的有效途径成为可能。































 京公网安备 11010802027423号
京公网安备 11010802027423号