当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Difluoromethylthiolator: A Toolbox of Reagents for Difluoromethylthiolation
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.accounts.1c00252 Jiang Wu 1 , Qilong Shen 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-06-29 , DOI: 10.1021/acs.accounts.1c00252 Jiang Wu 1 , Qilong Shen 1
Affiliation
|
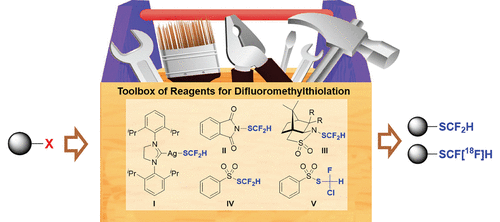
Recently, the strategic installation of a fluorine atom or a fluoroalkyl group site-selectively at the specific position of the target molecule has become a routine approach and daily practice for medicinal chemists in their endeavor to fine tune the structure of the lead compound to improve its physicochemical properties such as the cell membrane permeability and metabolic stability. Among many fluoroalkyl groups, the difluoromethylthio group (−SCF2H) has attracted recent intense attention. Largely due to the weak acidity of the proton in the difluoromethylthio group, the difluoromethylthio group is generally considered to be a lipophilic hydrogen-bonding donor and a bioisostere of the hydroxy/thio group that might interact with the heteroatom of the enzyme via a hydrogen bond to improve the binding selectivity of the drug molecule. Besides, the difluoromethylthio group is less lipophilic, less electron-withdrawing, and less stable to the acidic or basic environment than its analogue trifluoromethylthio group (−SCF3), making it easier to regulate the metabolic stability of drug molecules. These beneficial effects render the difluoromethylthio group one of the most favorable functional groups in drug design; consequently, there is an urgent need to develop new strategies for the efficient introduction of the difluoromethylthio group into small molecules under mild conditions. Over the last few decades, several different approaches to the preparation of difluoromethylthiolated compounds have been developed, including the difluoromethylation of thiolated substrates with an electrophilic/nucleophilic difluoromethylating reagent or the insertion of a difluoromethyl carbene into the S–H bond of the thiols. In contrast, we adopt an alternative approach to the preparation of difluoromethylthiolated compounds by late-stage direct difluoromethylthiolation of the specific substrates with a difluoromethylthiolating reagent. With this aim in mind, in the last 6 years we have successfully developed a toolbox of reagents that are capable of the direct introduction of the difluoromethylthio group into the target molecules, including nucleophilic difluoromethylthiolating reagent [(SIPr)AgSCF2H] I, electrophilic difluoromethylthiolating reagent PhthSCF2H II, three optically pure difluoromethylthiolating reagents camphorsultam-SCF2H III, radical difluoromethylthiolating reagent PhSO2SCF2H IV, and reagent PhSO2SCFClH V that could be used for the preparation of 18F-labeled [18F]ArSCF2H. These reagents reacted with a broad range of substrates to get access to difluoromethylthiolated compounds efficiently, thus providing medicinal chemists a powerful weapon for the direct introduction of the difluoromethylthio group into promising molecules during the search for new drugs.
中文翻译:

二氟甲基硫醇:二氟甲基硫醇化试剂的工具箱
最近,在目标分子的特定位置选择性地战略性安装氟原子或氟烷基已成为药物化学家努力微调先导化合物结构以改善其结构的常规方法和日常实践。理化性质,如细胞膜通透性和代谢稳定性。在许多氟烷基中,二氟甲硫基 (-SCF 2H)最近引起了强烈关注。很大程度上由于二氟甲硫基中质子的弱酸性,二氟甲硫基通常被认为是亲脂性氢键供体和羟基/硫基的生物等排体,可能通过氢键与酶的杂原子相互作用以提高药物分子的结合选择性。此外,二氟甲硫基比其类似物三氟甲硫基 (-SCF 3),更容易调控药物分子的代谢稳定性。这些有益效果使二氟甲硫基成为药物设计中最有利的官能团之一;因此,迫切需要开发在温和条件下将二氟甲硫基有效引入小分子的新策略。在过去的几十年里,已经开发了几种不同的制备二氟甲基硫醇化合物的方法,包括用亲电/亲核二氟甲基化试剂对硫醇化底物进行二氟甲基化,或将二氟甲基卡宾插入硫醇的 S-H 键。相比之下,我们采用了另一种方法来制备二氟甲硫基化合物,通过使用二氟甲硫基试剂对特定底物进行后期直接二氟甲硫基化。考虑到这一目标,在过去的 6 年中,我们成功开发了能够将二氟甲硫基直接引入目标分子的试剂工具箱,包括亲核二氟甲硫基化试剂 [(SIPr)AgSCF2 H]我,亲电difluoromethylthiolating试剂PhthSCF 2 ħ II,三种光学纯difluoromethylthiolating试剂樟脑磺-SCF 2 ħ III,自由基difluoromethylthiolating试剂PhSO 2 SCF 2 ħ IV,和试剂PhSO 2 SCFClH V可能被用于制备18 F-标记的[ 18 F]ArSCF 2H. 这些试剂与广泛的底物反应,可以有效地获得二氟甲硫基化合物,从而为药物化学家在寻找新药的过程中将二氟甲硫基直接引入有前景的分子提供了有力的武器。
更新日期:2021-07-20
中文翻译:

二氟甲基硫醇:二氟甲基硫醇化试剂的工具箱
最近,在目标分子的特定位置选择性地战略性安装氟原子或氟烷基已成为药物化学家努力微调先导化合物结构以改善其结构的常规方法和日常实践。理化性质,如细胞膜通透性和代谢稳定性。在许多氟烷基中,二氟甲硫基 (-SCF 2H)最近引起了强烈关注。很大程度上由于二氟甲硫基中质子的弱酸性,二氟甲硫基通常被认为是亲脂性氢键供体和羟基/硫基的生物等排体,可能通过氢键与酶的杂原子相互作用以提高药物分子的结合选择性。此外,二氟甲硫基比其类似物三氟甲硫基 (-SCF 3),更容易调控药物分子的代谢稳定性。这些有益效果使二氟甲硫基成为药物设计中最有利的官能团之一;因此,迫切需要开发在温和条件下将二氟甲硫基有效引入小分子的新策略。在过去的几十年里,已经开发了几种不同的制备二氟甲基硫醇化合物的方法,包括用亲电/亲核二氟甲基化试剂对硫醇化底物进行二氟甲基化,或将二氟甲基卡宾插入硫醇的 S-H 键。相比之下,我们采用了另一种方法来制备二氟甲硫基化合物,通过使用二氟甲硫基试剂对特定底物进行后期直接二氟甲硫基化。考虑到这一目标,在过去的 6 年中,我们成功开发了能够将二氟甲硫基直接引入目标分子的试剂工具箱,包括亲核二氟甲硫基化试剂 [(SIPr)AgSCF2 H]我,亲电difluoromethylthiolating试剂PhthSCF 2 ħ II,三种光学纯difluoromethylthiolating试剂樟脑磺-SCF 2 ħ III,自由基difluoromethylthiolating试剂PhSO 2 SCF 2 ħ IV,和试剂PhSO 2 SCFClH V可能被用于制备18 F-标记的[ 18 F]ArSCF 2H. 这些试剂与广泛的底物反应,可以有效地获得二氟甲硫基化合物,从而为药物化学家在寻找新药的过程中将二氟甲硫基直接引入有前景的分子提供了有力的武器。






























 京公网安备 11010802027423号
京公网安备 11010802027423号