Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting
ACS Nano ( IF 15.8 ) Pub Date : 2021-06-28 , DOI: 10.1021/acsnano.1c00616 Ping Wang 1 , Qingshuang Tang 1 , Lulu Zhang 1 , Menghong Xu 1 , Lihong Sun 1 , Suhui Sun 1 , Jinxia Zhang 1 , Shumin Wang 1 , Xiaolong Liang 1
ACS Nano ( IF 15.8 ) Pub Date : 2021-06-28 , DOI: 10.1021/acsnano.1c00616 Ping Wang 1 , Qingshuang Tang 1 , Lulu Zhang 1 , Menghong Xu 1 , Lihong Sun 1 , Suhui Sun 1 , Jinxia Zhang 1 , Shumin Wang 1 , Xiaolong Liang 1
Affiliation
|
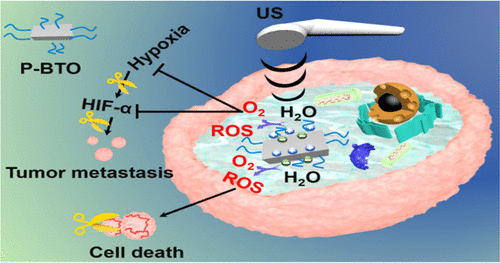
Hypoxia in a solid tumor microenvironment (TME) can lead to the overexpression of hypoxia-inducible factor-1α (HIF-1α), which correlates to tumor metastasis. Reactive oxygen species (ROS) induced tumor cell apoptosis is becoming a promising method in tumor treatment. Currently, the ROS generating systems, e.g., photodynamic treatment and sonodynamic treatment, highly depend on oxygen (O2) in the tumor microenvironment (TME). However, the level of O2 in TME is too low to produce enough ROS. Herein, we developed an ultrasmall DSPE-PEG2000 coated barium titanate nanoparticle (P-BTO) for tumor treatment based on ultrasound triggered piezocatalysis and water splitting. Interestingly, irradiated by ultrasound, the surface of ultasmall P-BTO nanoparticles produced imbalance charges, which induced a cascade of redox reaction processes to simultaneously generate ROS and O2, the latter one was hardly generated in large-sized barium titanate nanoparticles. The as-synthesized P-BTO reached the highest accumulation in the tumor site at 4 h after intravenous injection. The results showed that the produced O2 significantly alleviated the hypoxia of TME to down-regulate the expression of HIF-1α, and the produced ROS can efficiently kill tumor cells. Moreover, the tumor metastasis was also inhibited, providing a different way to treat triple-negative breast cancer, which was easily metastatic and lacked effective treatments in the clinic.
中文翻译:

超小钛酸钡纳米颗粒通过超声触发的压电催化和水分解用于高效缺氧肿瘤治疗
实体瘤微环境 (TME) 中的缺氧可导致缺氧诱导因子-1α (HIF-1α) 的过度表达,这与肿瘤转移相关。活性氧(ROS)诱导的肿瘤细胞凋亡正成为肿瘤治疗中一种很有前途的方法。目前,ROS产生系统,例如光动力治疗和声动力治疗,高度依赖于肿瘤微环境(TME)中的氧气(O 2)。然而,TME 中的 O 2水平太低,无法产生足够的 ROS。在此,我们开发了一种超小型 DSPE-PEG 2000涂层钛酸钡纳米颗粒 (P-BTO) 用于基于超声触发的压电催化和水分解的肿瘤治疗。有趣的是,超小尺寸的P-BTO纳米粒子在超声波照射下表面产生不平衡电荷,引发一系列氧化还原反应同时产生ROS和O 2,后者在大尺寸钛酸钡纳米粒子中几乎不产生。合成后的 P-BTO 在静脉注射后 4 小时在肿瘤部位达到最高蓄积。结果表明,产生的 O 2显着缓解TME缺氧下调HIF-1α的表达,产生的ROS可有效杀伤肿瘤细胞。此外,肿瘤转移也受到抑制,为治疗易转移、临床缺乏有效治疗手段的三阴性乳腺癌提供了一条不同的途径。
更新日期:2021-07-27
中文翻译:

超小钛酸钡纳米颗粒通过超声触发的压电催化和水分解用于高效缺氧肿瘤治疗
实体瘤微环境 (TME) 中的缺氧可导致缺氧诱导因子-1α (HIF-1α) 的过度表达,这与肿瘤转移相关。活性氧(ROS)诱导的肿瘤细胞凋亡正成为肿瘤治疗中一种很有前途的方法。目前,ROS产生系统,例如光动力治疗和声动力治疗,高度依赖于肿瘤微环境(TME)中的氧气(O 2)。然而,TME 中的 O 2水平太低,无法产生足够的 ROS。在此,我们开发了一种超小型 DSPE-PEG 2000涂层钛酸钡纳米颗粒 (P-BTO) 用于基于超声触发的压电催化和水分解的肿瘤治疗。有趣的是,超小尺寸的P-BTO纳米粒子在超声波照射下表面产生不平衡电荷,引发一系列氧化还原反应同时产生ROS和O 2,后者在大尺寸钛酸钡纳米粒子中几乎不产生。合成后的 P-BTO 在静脉注射后 4 小时在肿瘤部位达到最高蓄积。结果表明,产生的 O 2显着缓解TME缺氧下调HIF-1α的表达,产生的ROS可有效杀伤肿瘤细胞。此外,肿瘤转移也受到抑制,为治疗易转移、临床缺乏有效治疗手段的三阴性乳腺癌提供了一条不同的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号