Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2021-06-26 , DOI: 10.1016/j.colsurfb.2021.111945 Vamshi Krishna Rapalli 1 , Swati Sharma 1 , Aniruddha Roy 1 , Gautam Singhvi 1
|
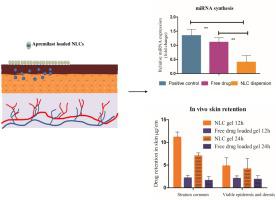
The present study aimed to develop Apremilast loaded nanostructured lipid carriers (NLCs) for topical delivery to overcome the limitations of oral therapy and increase the efficacy. Apremilast loaded NLCs were prepared by hot emulsification technique. The developed formulation was optimized by Box Behnken design and characterized for size, entrapment efficiency, and zeta potential. The selected formulation was investigated for in-vitro release, ex-vivo skin retention, dermatokinetic, psoriasis efficacy, in-vivo skin retention and skin irritation study. The NLCs characterization results showed its spherical shape with the particle size of 157.91 ± 1.267 nm (0.165 ± 0.017 PDI). The entrapment efficiency and zeta potential were found to be 69.144 ± 0.278% and -16.75 ± 1.40 mV, respectively. The in-vitro release study revealed a controlled release of Apremilast from NLCs up to 24 h. The ex-vivo study showed 3-fold enhanced skin retention compared to conventional gel preparation. The formulation depicted improved psoriasis efficacy indicating reduced TNF-α mRNA expression. The cytotoxicity and skin irritation study revealed the prepared formulation has no toxicity or irritation. The study depicts the NLCs loaded Apremilast can be explored for the topical delivery for treatment of psoriasis with improved skin retention and efficacy.
中文翻译:

用于局部给药的载有阿普司特纳米结构脂质载体的凝胶的设计和皮肤动力学评估:一种改善渗透和延长皮肤沉积的潜在方法
本研究旨在开发用于局部给药的载有阿普司特的纳米结构脂质载体 (NLC),以克服口服治疗的局限性并提高疗效。通过热乳化技术制备载有 Apremilast 的 NLC。开发的配方通过 Box Behnken 设计进行了优化,并表征了尺寸、截留效率和 zeta 电位。对选定的制剂进行体外释放、离体皮肤滞留、皮肤动力学、银屑病疗效、体内皮肤滞留和皮肤刺激研究。NLCs 表征结果显示其球形,粒径为 157.91 ± 1.267 nm (0.165 ± 0.017 PDI)。发现诱捕效率和 zeta 电位分别为 69.144 ± 0.278% 和 -16.75 ± 1.40 mV。体外释放研究揭示了 Apremilast 从 NLC 的受控释放长达 24 小时。离体研究表明,与传统凝胶制剂相比,皮肤保留能力提高了 3 倍。该制剂描述了改善的银屑病功效,表明降低的 TNF-α mRNA 表达。细胞毒性和皮肤刺激性研究表明制备的制剂没有毒性或刺激性。该研究描述了加载 Apremilast 的 NLC 可用于局部给药治疗银屑病,并具有改善的皮肤保留和功效。细胞毒性和皮肤刺激性研究表明制备的制剂没有毒性或刺激性。该研究描述了加载 Apremilast 的 NLC 可用于局部给药治疗银屑病,并具有改善的皮肤保留和功效。细胞毒性和皮肤刺激性研究表明制备的制剂没有毒性或刺激性。该研究描述了加载 Apremilast 的 NLC 可用于局部给药治疗银屑病,并具有改善的皮肤保留和功效。

































 京公网安备 11010802027423号
京公网安备 11010802027423号