当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2,3,4-Tetrahydroisoquinoline Derivatives as a Novel Deoxyribonuclease I Inhibitors
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-06-25 , DOI: 10.1002/cbdv.202100261 Mihajlo Gajić 1 , Budimir S Ilić 2 , Bojan P Bondžić 3 , Zdravko Džambaski 3 , Vesna V Kojić 4 , Dimitar S Jakimov 4 , Gordana Kocić 5 , Andrija Šmelcerović 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-06-25 , DOI: 10.1002/cbdv.202100261 Mihajlo Gajić 1 , Budimir S Ilić 2 , Bojan P Bondžić 3 , Zdravko Džambaski 3 , Vesna V Kojić 4 , Dimitar S Jakimov 4 , Gordana Kocić 5 , Andrija Šmelcerović 2
Affiliation
|
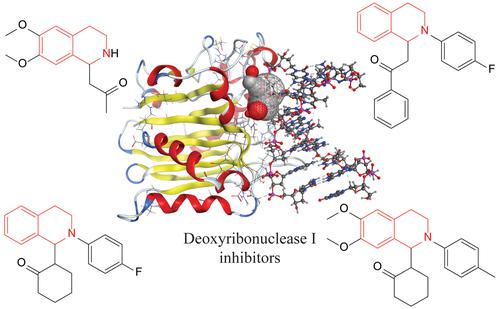
Herein we report an assessment of 24 1,2,3,4-tetrahydroisoquinoline derivatives for potential DNase I (deoxyribonuclease I) inhibitory properties in vitro. Four of them inhibited DNase I with IC50 values below 200 μM. The most potent was 1-(6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl)propan-2-one (2) (IC50=134.35±11.38 μM) exhibiting slightly better IC50 value compared to three other active compounds, 2-[2-(4-fluorophenyl)-1,2,3,4-tetrahydroisoquinolin-1-yl]-1-phenylethan-1-one (15) (IC50=147.51±14.87 μM), 2-[2-(4-fluorophenyl)-1,2,3,4-tetrahydroisoquinolin-1-yl]cyclohexan-1-one (18) (IC50=149.07±2.98 μM) and 2-[6,7-dimethoxy-2-(p-tolyl)-1,2,3,4-tetrahydroisoquinolin-1-yl]cyclohexan-1-one (22) (IC50=148.31±2.96 μM). Cytotoxicity assessment of the active DNase I inhibitors revealed a lack of toxic effects on the healthy cell lines MRC-5. Molecular docking and molecular dynamics simulations suggest that interactions with Glu 39, His 134, Asn 170, Tyr 211, Asp 251 and His 252 are an important factor for inhibitors affinity toward the DNase I. Observed interactions would be beneficial for the discovery of new active 1,2,3,4-tetrahydroisoquinoline-based inhibitors of DNase I, but might also encourage researchers to further explore and utilize potential therapeutic application of DNase I inhibitors, based on a versatile role of DNase I during apoptotic cell death.
中文翻译:

1,2,3,4-四氢异喹啉衍生物作为新型脱氧核糖核酸酶 I 抑制剂
在此,我们报告了对 24 1,2,3,4-四氢异喹啉衍生物的体外潜在 DNase I(脱氧核糖核酸酶 I)抑制特性的评估。其中四个抑制 DNase I,IC 50值低于 200 μM。最有效的是 1-(6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl)propan-2-one ( 2 ) (IC 50 =134.35±11.38 μM) 表现出稍好的 IC 50值与其他三种活性化合物相比,2-[2-(4-fluorophenyl)-1,2,3,4-tetrahydroisoquinolin-1-yl]-1-phenylethan-1-one ( 15 ) (IC 50 =147.51±14.87 μM),2-[2-(4-氟苯基)-1,2,3,4-四氢异喹啉-1-基]环己-1-酮 ( 18 ) (IC 50=149.07±2.98 μM) 和 2-[6,7-二甲氧基-2-( p -tolyl )-1,2,3,4-四氢异喹啉-1-基]环己-1-酮 ( 22 ) (IC 50=148.31±2.96 μM)。活性 DNase I 抑制剂的细胞毒性评估表明,对健康细胞系 MRC-5 没有毒性作用。分子对接和分子动力学模拟表明,与 Glu 39、His 134、Asn 170、Tyr 211、Asp 251 和 His 252 的相互作用是抑制剂对 DNase I 亲和力的重要因素。观察到的相互作用将有利于发现新的活性DNase I 的基于 1,2,3,4-四氢异喹啉的抑制剂,但也可能鼓励研究人员进一步探索和利用 DNase I 抑制剂的潜在治疗应用,这是基于 DNase I 在凋亡细胞死亡过程中的多功能作用。
更新日期:2021-08-17
中文翻译:

1,2,3,4-四氢异喹啉衍生物作为新型脱氧核糖核酸酶 I 抑制剂
在此,我们报告了对 24 1,2,3,4-四氢异喹啉衍生物的体外潜在 DNase I(脱氧核糖核酸酶 I)抑制特性的评估。其中四个抑制 DNase I,IC 50值低于 200 μM。最有效的是 1-(6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl)propan-2-one ( 2 ) (IC 50 =134.35±11.38 μM) 表现出稍好的 IC 50值与其他三种活性化合物相比,2-[2-(4-fluorophenyl)-1,2,3,4-tetrahydroisoquinolin-1-yl]-1-phenylethan-1-one ( 15 ) (IC 50 =147.51±14.87 μM),2-[2-(4-氟苯基)-1,2,3,4-四氢异喹啉-1-基]环己-1-酮 ( 18 ) (IC 50=149.07±2.98 μM) 和 2-[6,7-二甲氧基-2-( p -tolyl )-1,2,3,4-四氢异喹啉-1-基]环己-1-酮 ( 22 ) (IC 50=148.31±2.96 μM)。活性 DNase I 抑制剂的细胞毒性评估表明,对健康细胞系 MRC-5 没有毒性作用。分子对接和分子动力学模拟表明,与 Glu 39、His 134、Asn 170、Tyr 211、Asp 251 和 His 252 的相互作用是抑制剂对 DNase I 亲和力的重要因素。观察到的相互作用将有利于发现新的活性DNase I 的基于 1,2,3,4-四氢异喹啉的抑制剂,但也可能鼓励研究人员进一步探索和利用 DNase I 抑制剂的潜在治疗应用,这是基于 DNase I 在凋亡细胞死亡过程中的多功能作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号