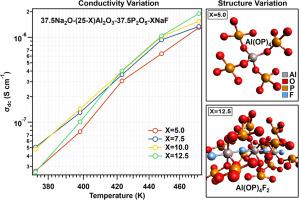
对用于钠离子电池的高离子导电固体电解质材料的需求已经大大增加。玻璃材料是高能钠离子电池的潜在固体电解质。然而,由于玻璃材料的低离子直流电导率和对可移动阳离子动力学的了解不足,因此对其在钠离子电池中的应用进行的有效研究尚无定论。在此,我们试图解决 NaF 替代 Al 2 O 3对 Na 3 Al 2 P 3 O 12 (NAP) 玻璃(摩尔%:37.5P 2 O 5 –25.0Al 2 O 3 –)电导率的影响37.5 钠2哦)。拉曼光谱显示取代基NaF浓度的增加显着影响Al 3+的配位以及钠离子在NAP玻璃网络结构中的分布。阻抗谱表明,随着 NaF 浓度的增加,NAP 玻璃的电导率变化高度依赖于特定的温度范围。在较低的温度范围 (<150 °C) 下,电导率最初随着 NaF 浓度的增加而增加,并且随着 NaF 浓度的进一步增加而降低;而在高于 150 °C 的温度下,电导率随着 NaF 浓度的增加而持续增加。已经使用基于 Summerfield 和 Ghosh 标度程序的时间-温度标度原理 (TTSP) 来分析与频率相关的交流电导率,以探测离子传导机制。包含低 NaF 浓度 (<7.5 mol%) 的 NAP 玻璃遵循 AC 电导率标度,IE.,它满足 TTSP 原则,关于基于 Summerfield 缩放程序的温度;这表明钠离子动力学与温度无关。包含高 NaF 浓度(> 10 mol%)的 NAP 玻璃的比例 AC 电导率曲线偏离了 Summerfield 比例,并进一步表明负责离子电导率的钠离子分数随着温度的升高而降低。使用 Ghosh 程序对所有玻璃样品的交流电导率曲线进行缩放表明,钠离子的数量密度和跳跃距离随温度升高而变化。改进的随机网络结构模型已进一步用于解释 NAP 玻璃电导率随 NaF 浓度增加的变化。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Understanding the sodium-ion dynamics in NASICON (Na3Al2P3O12) glass containing NaF: Scaling of electrical conductivity spectra
Quest for high ion-conducting solid electrolyte materials for sodium-ion batteries has been tremendously increasing. Glass materials are a potential solid electrolyte for high-energy Na-ion batteries. Nevertheless, the effective research on glass materials for their applications in Na-ion batteries is dawdling due to its low ionic DC-conductivity and poor understanding of the dynamics of mobile cations. Herein, we have attempted to address the effect of substitution of NaF for Al2O3 on the conductivity of Na3Al2P3O12(NAP) glass (mol%: 37.5P2O5–25.0Al2O3–37.5 Na2O). Raman spectra reveal that the increase in the substituent NaF concentration significantly affects the coordination of Al3+ and the distribution of sodium cations in the network structure of NAP glass. Impedance spectra reveal that the change in conductivity of NAP glass with an increase in the NaF concentration is highly dependent on a specific temperature range. At a lower temperature range, (<150 °C), the conductivity increases initially with the increase in NaF concentration and it decreases with any further increase in the NaF concentration; whereas, at temperatures above 150 °C, the conductivity increases continuously with an increase in the NaF concentration. The frequency-dependent AC-conductivity has been analyzed using the time-temperature scaling principle (TTSP) based on Summerfield and Ghosh scaling procedures to probe the ion conduction mechanism. The NAP glass that contains low NaF concentrations (<7.5 mol%) obeys the AC-conductivity scaling, i.e., it satisfies the TTSP principle, with respect to temperature based on the Summerfield scaling procedure; this indicates that the sodium-ion dynamics are independent of the temperature. The scaled AC-conductivity curves for NAP glass containing high NaF concentrations (>10 mol%) have deviated from Summerfield scaling and further indicate that the fraction of sodium-ions responsible for the ionic conductivity is decreasing with an increase in the temperature. Scaling of AC-conductivity curves for all the glass samples using the Ghosh procedure suggests that the number density along with the hopping distance of sodium ions changes with increasing temperature. The Modified random network structural model has been further utilized to explain the variation in the NAP glass conductivity with an increase in the NaF concentrations. The outcome of this work will certainly aid in designing the chemical compositions of glasses for the development of solid electrolyte materials for their applications in Na-ion batteries.





































 京公网安备 11010802027423号
京公网安备 11010802027423号