当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Allosteric Inhibition of Androgen Receptors by Binding Function 3 Antagonists from an Integrated Molecular Modeling Study
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-06-24 , DOI: 10.1021/acs.jcim.1c00124
Xiaotian Kong 1 , Enming Xing 1 , Tony Zhuang 2 , Pui-Kai Li 1 , Xiaolin Cheng 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-06-24 , DOI: 10.1021/acs.jcim.1c00124
Xiaotian Kong 1 , Enming Xing 1 , Tony Zhuang 2 , Pui-Kai Li 1 , Xiaolin Cheng 1
Affiliation
|
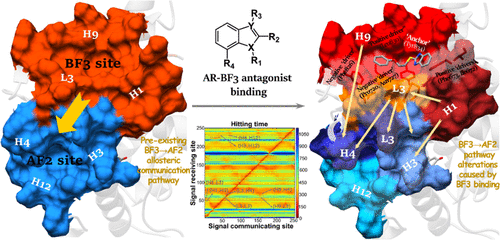
An androgen receptor (AR) is an intensively studied treatment target for castration-resistant prostate cancer that is irresponsive to conventional antiandrogen therapeutics. Binding function 3 (BF3) inhibitors with alternative modes of action have emerged as a promising approach to overcoming antiandrogen resistance. However, how these BF3 inhibitors modulate AR function remains elusive, hindering the development of BF3-targeting agents. Here, we performed an integrated computational study to interrogate the binding mechanism of several known BF3 inhibitors with ARs. Our results show that the inhibitory effect of the BF3 antagonists arises from their allosteric modulation of the activation function (AF2) site, which alters the dynamic coupling between the BF3 and AF2 sites as well as the AF2-coactivator (SRC2-3) interaction. Moreover, the per-residue binding energy analyses reveal the “anchor” role of the linker connecting the phenyl ring and benzimidazole/indole in these BF3 inhibitors. Furthermore, the allosteric driver-interacting residues are found to include both “positive”, e.g., Phe673 and Asn833, and “negative” ones, e.g., Phe826, and the differential interactions with these residues provide an explanation why stronger binding does not necessarily result in higher inhibitory activities. Finally, our allosteric communication pathway analyses delineate how the allosteric signals triggered by BF3 binding are propagated to the AF2 pocket through multiple short- and/or long-ranged transmission pathways. Collectively, our combined computational study provides a comprehensive structural mechanism underlying how the selected set of BF3 inhibitors modulate AR function, which will help guide future development of BF3 antagonists.
中文翻译:

综合分子建模研究中结合功能 3 拮抗剂对雄激素受体变构抑制的机制洞察
雄激素受体 (AR) 是一种深入研究的去势抵抗性前列腺癌治疗靶点,对常规抗雄激素疗法无反应。具有替代作用模式的结合功能 3 (BF3) 抑制剂已成为克服抗雄激素耐药性的一种有前途的方法。然而,这些 BF3 抑制剂如何调节 AR 功能仍然难以捉摸,阻碍了 BF3 靶向剂的开发。在这里,我们进行了一项综合计算研究,以询问几种已知的 BF3 抑制剂与 AR 的结合机制。我们的结果表明,BF3 拮抗剂的抑制作用源于它们对激活功能 (AF2) 位点的变构调节,这改变了 BF3 和 AF2 位点之间的动态耦合以及 AF2-共激活剂 (SRC2-3) 相互作用。而且,每个残基的结合能分析揭示了连接苯环和苯并咪唑/吲哚的接头在这些 BF3 抑制剂中的“锚定”作用。此外,发现变构驱动相互作用残基包括“阳性”残基,例如 Phe673 和 Asn833,以及“阴性”残基,例如 Phe826,与这些残基的不同相互作用解释了为什么不一定会产生更强的结合在更高的抑制活性。最后,我们的变构通信通路分析描绘了由 BF3 结合触发的变构信号如何通过多个短程和/或长程传输通路传播到 AF2 口袋。总的来说,我们的联合计算研究提供了一个全面的结构机制,为选定的一组 BF3 抑制剂如何调节 AR 功能提供了基础,
更新日期:2021-07-26
中文翻译:

综合分子建模研究中结合功能 3 拮抗剂对雄激素受体变构抑制的机制洞察
雄激素受体 (AR) 是一种深入研究的去势抵抗性前列腺癌治疗靶点,对常规抗雄激素疗法无反应。具有替代作用模式的结合功能 3 (BF3) 抑制剂已成为克服抗雄激素耐药性的一种有前途的方法。然而,这些 BF3 抑制剂如何调节 AR 功能仍然难以捉摸,阻碍了 BF3 靶向剂的开发。在这里,我们进行了一项综合计算研究,以询问几种已知的 BF3 抑制剂与 AR 的结合机制。我们的结果表明,BF3 拮抗剂的抑制作用源于它们对激活功能 (AF2) 位点的变构调节,这改变了 BF3 和 AF2 位点之间的动态耦合以及 AF2-共激活剂 (SRC2-3) 相互作用。而且,每个残基的结合能分析揭示了连接苯环和苯并咪唑/吲哚的接头在这些 BF3 抑制剂中的“锚定”作用。此外,发现变构驱动相互作用残基包括“阳性”残基,例如 Phe673 和 Asn833,以及“阴性”残基,例如 Phe826,与这些残基的不同相互作用解释了为什么不一定会产生更强的结合在更高的抑制活性。最后,我们的变构通信通路分析描绘了由 BF3 结合触发的变构信号如何通过多个短程和/或长程传输通路传播到 AF2 口袋。总的来说,我们的联合计算研究提供了一个全面的结构机制,为选定的一组 BF3 抑制剂如何调节 AR 功能提供了基础,

































 京公网安备 11010802027423号
京公网安备 11010802027423号