当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
QM/MM Study of Human Transketolase: Thiamine Diphosphate Activation Mechanism and Complete Catalytic Cycle
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-06-23 , DOI: 10.1021/acs.jcim.1c00190 Lionel Nauton 1 , Laurence Hecquet 1 , Vincent Théry 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-06-23 , DOI: 10.1021/acs.jcim.1c00190 Lionel Nauton 1 , Laurence Hecquet 1 , Vincent Théry 1
Affiliation
|
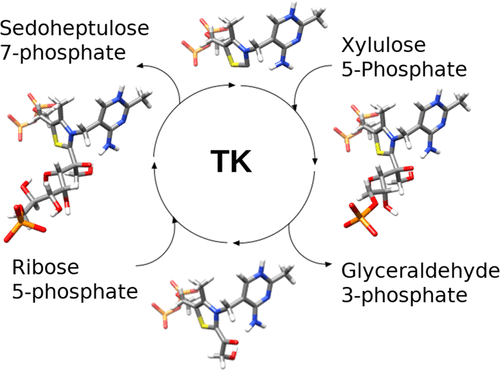
A computational model for human transketolase was proposed, showing that thiamine diphosphate activation was based on His110 in place of His481 reported in yeast transketolase. In addition, a complete catalytic reaction pathway was investigated using d-xylulose-5-phosphate and d-ribose-5-phosphate as substrates, showing at every step a perfect superimposition of our model with high-resolution crystallographic structures 3MOS, 4KXV, and 4KXX. This study shows that H2N4′ of the active thiamine diphosphate “V form” no longer has a self-activating role but allows self-stabilization of the cofactor and of the Breslow intermediate. These advances in our knowledge of the human transketolase mechanism offer interesting prospects for the design of new drugs, this enzyme being involved in several diseases, and for a better understanding of the reactions catalyzed by transketolases from other sources.
中文翻译:

人体转酮酶的 QM/MM 研究:硫胺素二磷酸激活机制和完整的催化循环
提出了人类转酮酶的计算模型,表明硫胺素二磷酸激活是基于 His110 代替酵母转酮酶中报道的 His481。此外,使用d -xylulose-5-phosphate 和d -xylulose-5-phosphate 研究了完整的催化反应途径-ribose-5-phosphate 作为底物,每一步都显示出我们模型与高分辨率晶体结构 3MOS、4KXV 和 4KXX 的完美叠加。该研究表明,活性硫胺素二磷酸“V 型”的 H2N4' 不再具有自激活作用,而是允许辅助因子和 Breslow 中间体的自稳定。我们对人类转酮酶机制知识的这些进展为新药的设计提供了有趣的前景,这种酶涉及多种疾病,并有助于更好地理解其他来源的转酮酶催化的反应。
更新日期:2021-07-26
中文翻译:

人体转酮酶的 QM/MM 研究:硫胺素二磷酸激活机制和完整的催化循环
提出了人类转酮酶的计算模型,表明硫胺素二磷酸激活是基于 His110 代替酵母转酮酶中报道的 His481。此外,使用d -xylulose-5-phosphate 和d -xylulose-5-phosphate 研究了完整的催化反应途径-ribose-5-phosphate 作为底物,每一步都显示出我们模型与高分辨率晶体结构 3MOS、4KXV 和 4KXX 的完美叠加。该研究表明,活性硫胺素二磷酸“V 型”的 H2N4' 不再具有自激活作用,而是允许辅助因子和 Breslow 中间体的自稳定。我们对人类转酮酶机制知识的这些进展为新药的设计提供了有趣的前景,这种酶涉及多种疾病,并有助于更好地理解其他来源的转酮酶催化的反应。













































 京公网安备 11010802027423号
京公网安备 11010802027423号