Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2021-06-23 , DOI: 10.1016/j.jclepro.2021.128074 Song Cheng , Yongzhi Liu , Baolin Xing , Xiaojing Qin , Chuangxiang Zhang , Hongying Xia
|
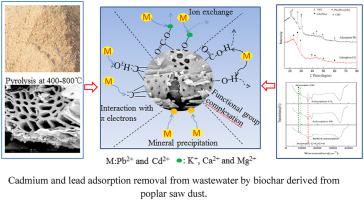
Biochar is produced at 400–800 °C by pyrolysis of poplar saw dust, which is used as the sustainable adsorbent to investigate the adsorption performance of Pb2+/Cd2+ from wastewater. The adsorption kinetics and isotherms of Pb2+/Cd2+ on biochar were determined. The maximum adsorption amount of Pb2+ is 62.68 mg/g, while that of Cd2+ is 49.32 mg/g at pH of 5 based on Langmuir equation. The Cd2+ adsorption was seriously inhibited, while the uptake of Pb(II) was not significantly affected in the multimetal system. The adsorption performance of Pb2+/Cd2+ was also investigated in different water systems. Mineral precipitation with Pb2+ is predominant mechanism for Pb2+ removal with the contribution proportion of 51.76%. Coordination with π electrons is the primary mechanism for Cd2+ adsorption, at 59.36% of the total adsorption capacity. Our findings provide a successful example of poplar saw dust converting into the sustainable adsorbent for Pb2+/Cd2+ removal from wastewater to implement the concept of “clean production”, which is worthy of further study.
中文翻译:

用杨木锯末衍生的可持续生物炭清洁废水中的铅和镉
Biochar 是在 400–800 °C 下通过杨木锯末热解生产的,用作可持续吸附剂来研究废水中 Pb 2+ /Cd 2+的吸附性能。测定了生物炭上Pb 2+ /Cd 2+的吸附动力学和等温线。根据朗缪尔方程,在pH值为5时,Pb 2+的最大吸附量为62.68 mg/g,而Cd 2+的最大吸附量为49.32 mg/g。Cd 2+吸附受到严重抑制,而多金属体系中Pb(II)的吸收没有显着影响。Pb 2+ /Cd 2+的吸附性能还在不同的水系统中进行了研究。矿物沉淀与铅2+是铅主要机制2+去除以51.76%的出资比例。与 π 电子的配位是 Cd 2+吸附的主要机制,占总吸附容量的 59.36%。我们的研究结果为杨木锯末转化为可持续吸附剂去除废水中的Pb 2+ /Cd 2+以实现“清洁生产”的概念提供了一个成功的例子,值得进一步研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号